Drugs, pharmaceuticals, medical devices made by Indians
m (Jyoti moved page Drugs/ Pharmaceuticals made by Indians to Drugs, pharmaceuticals, medical devices made by Indians without leaving a redirect) |
(→Cancer treatment) |
||
| Line 73: | Line 73: | ||
The institute is ready to transfer the technology for future development of curcumin as an anti-cancer treatment through animal and clinical trials. “The US patent adds value to our efforts to transfer technology and boosts the industry’s confidence in exploring validation and trials with international markets in mind,’’ Kishore said. | The institute is ready to transfer the technology for future development of curcumin as an anti-cancer treatment through animal and clinical trials. “The US patent adds value to our efforts to transfer technology and boosts the industry’s confidence in exploring validation and trials with international markets in mind,’’ Kishore said. | ||
| + | |||
| + | =Heart-valve technology= | ||
| + | == Transcatheter Aortic Valve Replacement== | ||
| + | [https://timesofindia.indiatimes.com/spotlight/made-in-india-heart-valve-technology-can-keep-your-heart-beating-strong/articleshow/73133882.cms January 7, 2020: ''The Times of India''] | ||
| + | |||
| + | |||
| + | ''' Made in India heart valve technology can keep your heart beating strong! ''' | ||
| + | |||
| + | Sarah*, a French woman was 83, when she had been advised that she had only a few hours to live. However, she decided to opt for a unique, cutting-edge procedure that could grant her a fresh lease of life. Within a year of the procedure, she traveled to America to achieve her dream of addressing a large gathering at a prestigious conference. Now, at age of 90, she continues to live an active and normal life. | ||
| + | |||
| + | ''' What threatened her life, and which procedure saved her? ''' | ||
| + | |||
| + | Sarah had a medical condition called Aortic Valve Stenosis (AS). The valve in her heart, which ensures normal blood-flow from the heart to the body, had narrowed with advancing age. This made it extremely difficult for her to carry out simple daily activities. She often felt out of breath. There was a tightness in her chest, frequently accompanied by pain. She felt dizzy even with brief movements. In short, living had become a struggle. | ||
| + | Medication did not provide any significant relief. The other available treatment was an open heart surgery for valve replacement. But that was an extremely risky option due to her age, the complex nature of the surgery, the long hospital stay, and high chance of complications post-surgery, especially during the recovery period of 10-12 months. Given these risks, surgical teams in Paris had refused to perform a surgical valve replacement. | ||
| + | At this point, Sarah consulted Dr. Alain Cribier, Professor emeritus at the Department of Cardiology, University Hospital Charles Nicolle in Rouen (France). Dr. Cribier suggested that she consider the option of an innovative non-surgical procedure called Transcatheter Aortic Valve Replacement (TAVR). | ||
| + | |||
| + | The procedure entailed a small cut in the groin or chest area to pass a catheter tube to replace the diseased valve with a bioprosthetic valve. There would be no need to cut open the chest cavity nor any need for general anesthesia as is usually required for surgery. The TAVR procedure would be performed over 30-40 minutes, and her recovery would be swift, requiring hospital stay of only 3 to 5 days. | ||
| + | |||
| + | Dr. Alain Cribier, who has established TAVR therapy worldwide shares, “In the 17 years since I performed the first TAVR procedure in 2002, more than 350,000 patients in 65 countries have undergone this revolutionary procedure. In the beginning, when the technology was new, TAVR was recommended only to high-risk patients who couldn’t undergo open heart surgery. However, advances in technology and availability of long-term safety data have resulted in TAVR becoming a routine and preferred procedure today. It can now be applied in a very high number of patients. In the year 2018, 68,000 TAVR procedures were performed in USA and 18,000 in France. In the hospital where I practice, we are conducting 5-10 procedures a week.” | ||
| + | |||
| + | |||
| + | However, the Indian scenario is quite different, as the procedure has not reached many patients who can benefit from it. Approximately 300,000 severe AS patients cannot undergo open-heart surgery because of old age, frailty, or other medical reasons. [1] TAVR therapy can be life saving for these patients. Yet, less than 1,000 TAVR procedures are being performed on an annual basis in India. Many lives can be saved if awareness of this promising procedure is raised. | ||
| + | |||
| + | |||
| + | Meril Life Sciences, a Gujarat-based medical device manufacturer with a global footprint spread over 150 countries, is committed to bring about this change. Known for indigenous research and development, and a broad portfolio of stents, surgical and orthopedic devices, this company has recently launched an advanced bioprosthetic valve. The new valve has a unique ‘hybrid honeycomb’ design that allows doctors to precisely place the device at its natural position. This reduces the need for a new pacemaker, otherwise required in 10 to 30 percent of patients receiving other available valve technologies. | ||
| + | |||
| + | Results of the MyVal-1 scientific study , published in the Euro Intervention Journal, prove the benefits of this valve technology. There was high device success, low incidence of stroke, and low requirement of a permanent pacemaker implant post-procedure. Significant improvement in patient heart function and quality of life was also observed. After CE approval of this valve technology, hospitals in Europe have commenced using the valve due to these benefits. | ||
| + | |||
| + | |||
| + | “Companies like Meril Life Sciences are helping bring the benefits of non-surgical TAVR therapy to more patients by training Interventional Cardiologists in India. They have also made this cutting-edge treatment more accessible and more affordable to Indian patients,” sums up Dr. Alain Cribier. | ||
| + | |||
| + | As these efforts come to fruition, many more Indian patients will have the chance to live life to their heart’s content, just as Sarah did. | ||
| + | |||
| + | |||
| + | * Patient name edited to ensure privacy. | ||
| + | |||
| + | [1] Based on incidence data applied to the Indian population, J Am Coll Cardiol. 2013;62(11):1002-1012. doi:10.1016/j.jacc.2013.05.015 | ||
| + | |||
| + | Note: This article is intended for educational purposes only, and does not seek to serve as a substitute for medical advice, nor to advocate a treatment or device in use for patients. Only qualified medical experts can provide advice regarding your individual treatment. Please consult your doctor for more details. | ||
[[Category:Economy-Industry-Resources|D DRUGS/ PHARMACEUTICALS MADE BY INDIANSDRUGS/ PHARMACEUTICALS MADE BY INDIANS | [[Category:Economy-Industry-Resources|D DRUGS/ PHARMACEUTICALS MADE BY INDIANSDRUGS/ PHARMACEUTICALS MADE BY INDIANS | ||
| − | DRUGS | + | DRUGS, PHARMACEUTICALS, MEDICAL DEVICES MADE BY INDIANS]] |
[[Category:Health|D DRUGS/ PHARMACEUTICALS MADE BY INDIANSDRUGS/ PHARMACEUTICALS MADE BY INDIANS | [[Category:Health|D DRUGS/ PHARMACEUTICALS MADE BY INDIANSDRUGS/ PHARMACEUTICALS MADE BY INDIANS | ||
| − | DRUGS | + | DRUGS, PHARMACEUTICALS, MEDICAL DEVICES MADE BY INDIANS]] |
[[Category:India|D DRUGS/ PHARMACEUTICALS MADE BY INDIANSDRUGS/ PHARMACEUTICALS MADE BY INDIANS | [[Category:India|D DRUGS/ PHARMACEUTICALS MADE BY INDIANSDRUGS/ PHARMACEUTICALS MADE BY INDIANS | ||
| − | DRUGS | + | DRUGS, PHARMACEUTICALS, MEDICAL DEVICES MADE BY INDIANS]] |
| − | [[Category:Pages with broken file links|DRUGS | + | [[Category:Pages with broken file links|DRUGS, PHARMACEUTICALS, MEDICAL DEVICES MADE BY INDIANS]] |
[[Category:S&T|D DRUGS/ PHARMACEUTICALS MADE BY INDIANSDRUGS/ PHARMACEUTICALS MADE BY INDIANS | [[Category:S&T|D DRUGS/ PHARMACEUTICALS MADE BY INDIANSDRUGS/ PHARMACEUTICALS MADE BY INDIANS | ||
| − | DRUGS | + | DRUGS, PHARMACEUTICALS, MEDICAL DEVICES MADE BY INDIANS]] |
=Insulin= | =Insulin= | ||
Revision as of 20:37, 17 September 2022
This is a collection of articles archived for the excellence of their content. |
Contents |
Cancer detection
2019/ Test to detect spread or relapse
Umesh Isalkar, August 21, 2019: The Times of India
A simple blood test costing Rs 15,000 will soon help doctors detect the spread or relapse of cancer in patients, helping them to plan an efficient treatment module. The test, based on a liquid biopsy technology called OncoDiscover, will help pick up circulating cancer cells in a patient’s blood much before they have affected or spread to other body parts.
Developed by Pune-based scientist Jayant Khandare, the diagnostic tool may also be used to plan treatment or to assess how well the existing treatment regime is working. “The test will be launched in a phased manner all over India from September,” Khandare told TOI.
‘Indian cancer test kit will be far cheaper than the US version’
The long-term plan is to release the test in key global markets within the next few years,” Khandare told TOI.
Khandare is the chief scientific officer at Actorius Innovations and Research Pvt Ltd, a startup co-founded by him and Aravindan Vasudevan in 2013. The startup has been funded for high-risk innovation by the Biotechnology Industry Research Assistance Council, an industry support wing of the department of biotechnology, Union ministry of science and technology.
Khandare said the technology was among the few laboratory-to-clinic translational researches, a rarity in India. “OncoDiscover can allow regular monitoring of disease progression. It can also help in providing an early indication for cancer relapse. It is a blood test that detects circulating tumour cells even when their presence is extremely low or minuscule in a patient’s blood,” he said.
The Central Drugs Standard Control Organisation, the regulatory body for drugs and medical devices, has already granted a licence for the manufacture and sale of the test kit in India under the Medical Device Rules, 2017.
Khandare says it is the second such technology in the world, after the one already available in the US. The Indian version would be far cheaper and more specific than the US one, he said. “CellSearch, the US test for the detection of circulating tumour cells has been sold to Menarini Biotech by Johnson & Johnson. It has been available in the US since 2004 and has the approval of the US Food and Drug Administration. The test costs between Rs 84,000 and Rs 1 lakh and is not available in India,” Khandare said.
Cancer treatment
Cytotron/ 2019
Chethan Kumar, Nov 12, 2019: The Times of India
The US Food and Drug Administration (FDA)’s Centre for Devices and Radiological Health has designated a medical invention by a Bengaluru-based scientist as a “breakthrough device” in liver, pancreatic and breast cancer treatment.
Cytotron, developed by Rajah Vijay Kumar, aids in tissue engineering of cancer cells, altering how specific proteins are regulated to stop these cells from multiplying and spreading.
“We are pleased to inform you that your device and proposed indication for use meet the criteria and have been granted designation as a breakthrough device,” states a communique from the FDA wing to Shreis Scalene Sciences, the company that had taken the device to the US.
‘Anti-cancer device to be manufactured in India’
Cytotron is intended to cause degeneration of uncontrolled growth of tissues. “It is indicated for treating protein-linked, abnormally regenerating disorders such as neoplastic disease, and allowing extended progression free survival, with pain relief, palliation, improved quality and dignity of life,” says the letter.
Rajah Vijay Kumar had developed Cytotron at the Centre for Advanced Research and Development, which is headquartered in Bhopal, after nearly 30 years of research into cellular pathways and interactions with specifically modulated fast radio bursts.
“It is a great feeling that after so many years of hard work, against all odds, an institution like the USFDA is designating our work as a breakthrough in the treatment of three types of cancers,” Kumar said.
New technologies in the battle against cancer have generally been hard to come by. It’s even rarer for an Indian device to get breakthrough status in the US. The Centre for Devices and Radiological Health is responsible for pre-market approval of all medical devices in the US, ensuring they are safe for use and effective.
“The devices will all be made in India, given that there are hardly any imported components. And our American partner will take the device to the US. Cytotron is already an approved medical device and is in use in the UAE, Mexico, Malaysia and Hong Kong, among others,” Kumar said.
Turmeric- based curcumin
Rajiv G, January 14, 2020: The Times of India
THIRUVANANTHAPURAM: A potentially breakthrough cancer-fighting technology involving a molecule extracted from turmeric has won Thiruvananthapuram’s Sree Chitra Tirunal Institute for Medical Sciences a US patent.
According to Lissy Krishnan, head of Sree Chitra’s research team, delivery of “curcumin” directly to the affected tissues rather than through conventional oral or intravenous methods enables it to target malignant cancer cells while sparing the healthy ones around them.
Turmeric has proven anti-cancer properties and curcumin, a molecule extracted from it, is easily absorbed by the body and aids blood clotting, Krishnan said. At Sree Chitra, research funded by the Indian Council for Medical Research focused on processing curcumin to form a easy-to-use wafer configuration. When applied to body tissues, the curcumin present in the wafer is released into tissue fluids.
Human albumin, or rich proteins present in the fibrin clot produced from heavy bleeding, binds albumin receptors to cancer cells, thereby permitting its entry into the cells. Simultaneously, the fibrin clot is removed by the body’s natural clot breakdown mechanism without any adverse effect.
“The fibrin wafer is targeted for implantation into the affected site after surgical removal of cancer tissue for killing any remaining malignant cells that have the potential to cause recurrence of cancer or spread to other parts of the body. In addition to drug delivery, the fibrin wafer can promote blood clotting at the surgical site,” said Asha Kishore, director of Sree Chitra.
The institute is ready to transfer the technology for future development of curcumin as an anti-cancer treatment through animal and clinical trials. “The US patent adds value to our efforts to transfer technology and boosts the industry’s confidence in exploring validation and trials with international markets in mind,’’ Kishore said.
Heart-valve technology
Transcatheter Aortic Valve Replacement
January 7, 2020: The Times of India
Made in India heart valve technology can keep your heart beating strong!
Sarah*, a French woman was 83, when she had been advised that she had only a few hours to live. However, she decided to opt for a unique, cutting-edge procedure that could grant her a fresh lease of life. Within a year of the procedure, she traveled to America to achieve her dream of addressing a large gathering at a prestigious conference. Now, at age of 90, she continues to live an active and normal life.
What threatened her life, and which procedure saved her?
Sarah had a medical condition called Aortic Valve Stenosis (AS). The valve in her heart, which ensures normal blood-flow from the heart to the body, had narrowed with advancing age. This made it extremely difficult for her to carry out simple daily activities. She often felt out of breath. There was a tightness in her chest, frequently accompanied by pain. She felt dizzy even with brief movements. In short, living had become a struggle. Medication did not provide any significant relief. The other available treatment was an open heart surgery for valve replacement. But that was an extremely risky option due to her age, the complex nature of the surgery, the long hospital stay, and high chance of complications post-surgery, especially during the recovery period of 10-12 months. Given these risks, surgical teams in Paris had refused to perform a surgical valve replacement. At this point, Sarah consulted Dr. Alain Cribier, Professor emeritus at the Department of Cardiology, University Hospital Charles Nicolle in Rouen (France). Dr. Cribier suggested that she consider the option of an innovative non-surgical procedure called Transcatheter Aortic Valve Replacement (TAVR).
The procedure entailed a small cut in the groin or chest area to pass a catheter tube to replace the diseased valve with a bioprosthetic valve. There would be no need to cut open the chest cavity nor any need for general anesthesia as is usually required for surgery. The TAVR procedure would be performed over 30-40 minutes, and her recovery would be swift, requiring hospital stay of only 3 to 5 days.
Dr. Alain Cribier, who has established TAVR therapy worldwide shares, “In the 17 years since I performed the first TAVR procedure in 2002, more than 350,000 patients in 65 countries have undergone this revolutionary procedure. In the beginning, when the technology was new, TAVR was recommended only to high-risk patients who couldn’t undergo open heart surgery. However, advances in technology and availability of long-term safety data have resulted in TAVR becoming a routine and preferred procedure today. It can now be applied in a very high number of patients. In the year 2018, 68,000 TAVR procedures were performed in USA and 18,000 in France. In the hospital where I practice, we are conducting 5-10 procedures a week.”
However, the Indian scenario is quite different, as the procedure has not reached many patients who can benefit from it. Approximately 300,000 severe AS patients cannot undergo open-heart surgery because of old age, frailty, or other medical reasons. [1] TAVR therapy can be life saving for these patients. Yet, less than 1,000 TAVR procedures are being performed on an annual basis in India. Many lives can be saved if awareness of this promising procedure is raised.
Meril Life Sciences, a Gujarat-based medical device manufacturer with a global footprint spread over 150 countries, is committed to bring about this change. Known for indigenous research and development, and a broad portfolio of stents, surgical and orthopedic devices, this company has recently launched an advanced bioprosthetic valve. The new valve has a unique ‘hybrid honeycomb’ design that allows doctors to precisely place the device at its natural position. This reduces the need for a new pacemaker, otherwise required in 10 to 30 percent of patients receiving other available valve technologies.
Results of the MyVal-1 scientific study , published in the Euro Intervention Journal, prove the benefits of this valve technology. There was high device success, low incidence of stroke, and low requirement of a permanent pacemaker implant post-procedure. Significant improvement in patient heart function and quality of life was also observed. After CE approval of this valve technology, hospitals in Europe have commenced using the valve due to these benefits.
“Companies like Meril Life Sciences are helping bring the benefits of non-surgical TAVR therapy to more patients by training Interventional Cardiologists in India. They have also made this cutting-edge treatment more accessible and more affordable to Indian patients,” sums up Dr. Alain Cribier.
As these efforts come to fruition, many more Indian patients will have the chance to live life to their heart’s content, just as Sarah did.
- Patient name edited to ensure privacy.
[1] Based on incidence data applied to the Indian population, J Am Coll Cardiol. 2013;62(11):1002-1012. doi:10.1016/j.jacc.2013.05.015
Note: This article is intended for educational purposes only, and does not seek to serve as a substitute for medical advice, nor to advocate a treatment or device in use for patients. Only qualified medical experts can provide advice regarding your individual treatment. Please consult your doctor for more details.
Insulin
That can be kept without refrigeration/ 2021
Jhimli MukherjeePandey, Sep 24, 2021: The Times of India
From: Jhimli MukherjeePandey, Sep 24, 2021: The Times of India
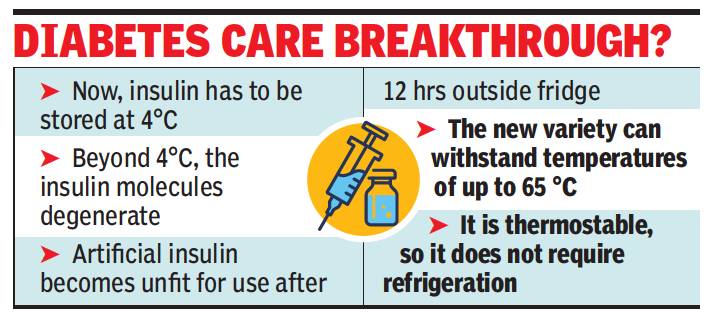
A team of scientists, including two from Kolkata, have developed a “thermostable” variety of insulin, which eliminates the need to keep it refrigerated. The development is being seen as a breakthrough in scientific circles, with portability being the biggest hurdle for insulin-dependent diabetics.
The research has been led by two scientists of the Bose Institute and the Indian Institute of Chemical Biology (IICB) here and two others from the Indian Institute of Chemical Technology (IICT), Hyderabad.
“You will be able to keep it outside the refrigerator for as long as you want, something that will help diabetes patients across the world because carrying insulin along with them was considered impossible all this while,” said Subhrangsu Chatterjee, a faculty member at Bose Institute.
“Though for the moment we are calling it ‘insulock’, we are in the process of appealing to the department of science and technology (DST) to name it after Acharya Jagadish Chandra Bose,” he added.
The research has been lauded by iScience, a science journal of international repute. Chatterjee and Partha Chakrabarti, a faculty member at IICB, along with IICT’s B Jagadeesh and J Reddy, were able to introduce a matrix of four amino acid peptide molecules inside insulin molecules, which prevented solidification of the insulin molecules even when not refrigerated. While insulin needs to be kept at an ideal temperature of 4 °C now, this new variety would be able to withstand a temperature of up to 65 °C, the researchers claim. The four-year-long research into the structural design of insu-lock was funded jointly by DST and CSIR.
Chatterjee said, after 12 hours of staying at normal room temperature, it reaches a stage where it becomes unfit for use. “That is why it is so expensive. We are hopeful that DST and CSIR will now help us go for corporate tie-ups for mass production,” he told TOI.
Trientine/ D-Pencillamine
MSN Laboratories/ 2019
Over two years after patients of a rare genetic condition called Wilson’s Disease suffered due to acute shortage of a life-saving drug called D-Pencillamine across the country, there is finally a reason to cheer. An Indian company has begun manufacturing a drug called Trientine, which is considered superior to D-Pencillamine due to fewer side-effects, at a fraction of its international price.
What makes this new drug launch an emotional event is the behind-the-scenes lobbying by a group of parents, an NGO and a doctor from Mumbai who worked to convince Hyderabad-based MSN Laboratories to take up the manufacture of Trientine.
At a time when stories of the greed of pharmaceutical companies abound, this is a positive instance of pharmaceutical support. Dr Aabha Nagral, a hepatologist who set up NGO Children’s Liver Foundation, said a couple of children had to stop treatment when the drug shortage occurred in 2016.
‘Firm involved in social work; not driven by profits’
We organised a picnic for our patient group where two youngsters shared that they had no drugs at that time and were worried about the future,” said Dr Nagral, who has treated approximately 100 Wilson’s Disease patients in her care at any given time.
The Ranes from Vile Parle, too, recall how their daughter Gauri was hospitalised a few times during the shortage. “Fluid would get accumulated in her abdomen, requiring hospitalisation. It was a traumatising time as her liver was failing quickly,” said Satish Rane. Luckily, she got a cadaveric liver donation and is doing well since the transplant a year ago.
Rajesh S (name changed), a 45-year-old research scientist, was diagnosed with Wilson’s Disease a year back. “We had heard of shortages and expensive medicines costing almost Rs 1 lakh for 100 tablets but we now pay Rs 16,000 for 100 tablets,” said his wife.
The turnaround took months of gathering information and planning. After the shortage was fixed by the Union government’s directive to pharmaceutical companies in August 2016, Dr Nagral and the patient group actively started to seek information about Trientine. They heard from pharmaceutical agents that the active ingredient for Trientine was manufactured in an Indian company and exported to multinational companies located overseas.
“We applied through RTI to the Union ministry of information on the manufacture of Trientine and D-Pencillamine, but got little,” said Dr Nagral. That is when they began to directly appeal to pharmaceutical companies for help. MSN Laboratories, which has been manufacturing active pharmaceutical ingredients for export purposes for years, decided to help out. Ajit Bhadauria of MSN Laboratories said the company didn’t take up the manufacture of Trientine for profits. “It is purely a part of our social work as a pharmaceutical company,” he said.
Vaccines
Pneumonia vaccine/ 2020
Sushmi Dey, India gets its first desi pneumonia vaccine, July 16, 2020: The Times of India
NEW DELHI: India has got its first locally developed pneumonia vaccine, reducing its dependence on imports for immunisation against a disease that contributes to most deaths in children under five years of age. The Drugs Controller General of India (DCGI) on Tuesday approved Pneumococcal Polysaccharide Conjugate Vaccine developed by Serum Institute of India. The Pune-based company can now manufacture the vaccine in India for use.
This vaccine is used for active immunisation against invasive disease and pneumonia caused by “streptococcus pneumonia” in infants. “Having an indigenous pneumococcal vaccine will be a game-changer in our endeavour to reduce child mortality. Pneumonia is the most important cause of child deaths, and pneomococci are responsible for half of serious pneumonias. India’s vaccine is a boon for our country and the world,” says Dr V K Paul, member -health at NITI Aayog.
India accounted for the second highest number of deaths in under-five children in 2018 because of pneumonia. According to Unicef, India reported 1,27,000 under five deaths due to pneumonia in 2018. Earlier, the demand of this vaccine was met substantially by licensed importers in the country since the manufacturers were all vaccine companies based outside. The regulatory approval is based on clinical evidence secured by the company after conducting Phase I, Phase II and Phase III clinical trials of the vaccine. Serum Institute has conducted these trials in India as well as in Gambia.
While immunisation against the disease has improved in India led by government initiatives and awareness programmes, many children — mainly female children — are still left out of the coverage. Experts say a locally made vaccine is likely to make the vaccine more accessible and affordable.
India accounted for the second highest number of deaths in under-five children in 2018 because of pneumonia. According to Unicef, India reported 1,27,000 under five deaths due to pneumonia in 2018.
Covaxin/ 2021
Swati Bharadwaj, January 23, 2021: The Times of India
Amid efficacy row, Covaxin gets thumbs-up from Lancet
HYDERABAD: Amid a raging controversy over the efficacy of Covaxin, India’s first indigenous Covid-19 vaccine, reputed medical journal the Lancet Infectious Diseases said the vaccine produced tolerable safety outcomes and enhanced immune responses in its Phase 1 trials.
With this, Covaxin has become the first Covid-19 vaccine from India to have its data published in Lancet, said Suchitra Ella, joint managing director of Bharat Biotech, which is developing the vaccine in association with the Indian Council for Medical Research and the National Institute of Virology, Pune.
"BBV152 (codename for Covaxin) induced binding and neutralising antibody responses and with the inclusion of the Algel-IMDG adjuvant, this is the first inactivated SARS-CoV-2 vaccine that has been reported to induce a Th1-biased response," said the journal.
Covaxin was well tolerated in all dose groups, with no vaccine-related serious adverse events and the sole serious adverse event was not causally associated with the vaccine, the journal said.
According to biotech industry experts, any vaccine causes some discomfort like pain or fever but it is said to be tolerable when it is resolved without any treatment and is considered safe. A serious adverse side-effect is one which takes a lot of time and effort to treat and causes partial damage to the subject.
The biggest criticism against Covaxin was that none of its data was in the public domain when it was given emergency-use authorisation. Sources said Bharat Biotech is hoping to counter the critics with the Lancet piece.
Terming it as "international validation of Indian innovation", Ella tweeted: "Extremely proud to announce Phase 1 clinical trial studies of Covaxin published in the most prestigious medical journal of The Lancet Infectious Diseases."
Ella also said that 13,000 volunteers (out of 25,800) have been successfully administered the second dose of Covaxin in Phase 3 trials.
Lancet said all adverse events observed in the Phase 1 trials were mild or moderate and were more frequent after the first dose than the second. No significant differences were observed between the vaccinated and control groups, it added.
The most common adverse event was pain at the injection site, followed by headache, fatigue and fever. "The overall incidence of solicited local and systemic adverse events in this study was 14-21% in all vaccine-treated groups, which is noticeably lower than the rates for other SARS-CoV-2 vaccine platform candidates," it said.
As many as 44 unsolicited adverse events were reported by 24 (6%) of the 375 participants. The healthy volunteers, in the 18-55 years age group that were enrolled in the trials conducted in July-August 2020, were administered the two doses 14 days apart.
Of those enrolled, 100 each were randomly assigned to the three vaccine groups, and 75 were randomly assigned to the control group (Algel only).
Apart from carrying Covaxin Phase 1 trial data, the Lancet carried another article saying that the indigenous Indian vaccine was a "welcome addition to the Covid-19 vaccine landscape". But it also said that it would wait for Phase 3 trial results.
Pointing out that mathematical models indicate there will not be an adequate supply of vaccines available to cover the global population until 2023, the article said: "Bharat Biotech, experienced with developing and distributing vaccines to LMICs (low & middle-income countries) is poised to bridge the vaccine disparity gap using the well-established inactivated whole-virus vaccine platform."
However, it said that until questions about BBV152’s efficacy and ability to subvert a Th2 response are answered by a more diverse multinational Phase 3 trial to comprehensively assess efficacy and long-term safety, "we will wait with cautious optimism on this vaccine candidate poised to bolster worldwide equitable access to Covid-19 prevention".