Drugs and Pharmaceuticals: India
This is a collection of articles archived for the excellence of their content. Readers will be able to edit existing articles and post new articles directly |
This is a collection of articles archived for the excellence of their content. |
Active pharmaceutical ingredients’ (API) prices

Active pharmaceutical ingredients imported from China, 2016
The Times of India
See graphic, The dependence of the Indian pharmaceutical industry on ingredients imported from China Active pharmaceutical ingredients imported from China, 2016
2020- February 2023
Rupali Mukherjee, February 27, 2023: The Times of India
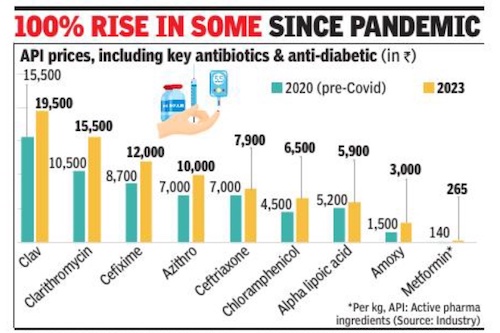
From: Rupali Mukherjee, February 27, 2023: The Times of India
Mumbai : A steep price increase of over 100% from prepandemic levels of certain raw materials for essential drugs — called active pharmaceutical ingredients (API) — has raised eyebrows in the industry. Interestingly, the sharp hike — which should have corrected by now with the improvement in supply chain and logistics — has been witnessed in high-volume key antibiotics including azithromycin and amoxicillin imported from China. These are also the products where India has near or complete dependence on China, industry experts told TOI . As against this, prices of most vitamins, including vitamin B and D, also imported from China, are at an all-time low.
Besides APIs for key antibiotics, anti-TB rifampicin and anti-diabetes metformin have also doubled from the pre-pandemic level, they added. Antibiotics including Azithro, Clav and Amoxicillin are high-volume products, the production of which is dependent on imports from the neighbouring country.
The dramatic increase is being attributed to multiple reasons like inflation, increase in prices of key starting materials and solvents due to crude, and the freight cost, experts added. Further, fewagents have been controlling the imports of certain products, which has led to a cartelisation of sorts. The government should break the monopoly and prevent sole agents from controlling the market, an executive with a firm manufacturing APIs said. A Mumbai-based trader stated, “It’s more about Chinese fleecing as we are dependent on them, coupled with some registration agents pushing themto form unholy cartels for their own benefits. ”
It may be noted that cost has gone up for only those drugs for which raw materials are imported from China. Once the pandemic spread, API prices of certain drugs like fever and pain relief medication, paracetamol, life-saving antibiotic Meropenem (also used for Covid), and antidiabetic metformin, jumped by over 200%, putting the spotlight back on India’s near total-dependence on China. Since 2020, prices have been increasing due to the pandemicinduced supply disruption and logistics challenges, pinching the industry hard. “API prices have remained inflated due to the lockdown in China for the past twothree years, logistics and high energy prices. So far, companies have been managing API prices by being efficient with operations. Also, the government had allowed some price revisions a couple of years ago,” said Sujay Shetty, global health industries advisory leader at PwC India.
According to pharma policy, for essential drugs, prices undergo a change — either increase or decrease — in line with the annual wholesale price index (WPI) in April each year. Non-scheduled drugs (those outside price control) are allowed an annual increase of 10% every year.
Advertisements, inappropriate
Non-enforcement of Rule 170 of Drugs and Cosmetics Act questioned by SC/ 2024
Anonna Dutt, Sep 2, 2024: The Indian Express
Justices Hima Kohli and Sandeep Mehta, while hearing the ongoing Supreme Court case against Patanjali Ayurved, pulled up the AYUSH ministry for its July 1 notification asking state licensing authorities “not to initiate/take any action under” Rule 170 of the Drugs and Cosmetics Act.
The rule, introduced in 2018, is designed to prevent misleading advertisements of AYUSH products. The AYUSH ministry’s July 1 notification reiterated its position made in a previous letter, dated August 29, 2023.
What is Rule 170?
In 2018, the government brought in Rule 170 to govern the manufacture, storage, and sale of medicines in the country, “specifically for controlling inappropriate advertisements of Ayurvedic, Siddha and Unani medicines”.
The rule prohibits AYUSH drug manufacturers from advertising their products without approval and allotment of a unique identification number from the state licensing authority. The manufacturers have to submit details such as textual references and rationale for the medicine from authoritative books, indication for use, evidence of safety, effectiveness, and quality of drugs.
The rule states that the application will be rejected if the manufacturer does not provide their contact details, if the contents of the advertisement are obscene or vulgar, products for enhancement of male or female sexual organs, depicts photographs or testimonials from celebrities or government officials, refers to any government organisation, gives false impression or makes misleading or exaggerated claims.
The rule was introduced after a parliamentary standing committee highlighted the problem of misleading claims, and the need for the AYUSH ministry to proactively pursue the issue.
What are challenges to regulate AYUSH drugs?
Like allopathic medicines, manufacturers of AYUSH drugs also have to seek a license from the drug controller. As per the Drugs and Cosmetics Act, phase I, II, and III trials for approval of new allopathic medicines or equivalence studies for generic versions have to be conducted before a drug is cleared for marketing.
However, such trials are not necessary for AYUSH drugs. According to the aforementioned act, most AYUSH drugs can be approved simply based on the rationale provided in authoritative texts of that particular stream. Safety trials have to be conducted only for formulations that use around 60 specific ingredients listed in the act, such as snake venom, snake head, heavy metals such as arsenic and mercury, and compounds such as copper sulphate.
For licensing of drugs containing these ingredients and traditional drugs used for new indications, proof of effectiveness has to be provided as per the Act.
Why did AYUSH ministry direct licensing authorities to ignore the rule?
The Ayurvedic, Siddha and Unani Drugs Technical Advisory Board (ASUDTAB), an expert body that recommends actions relating to regulation of AYUSH drugs, in a meeting in May 2023 said that Rule 170 could be omitted as amendments in Drugs and Magic Remedies Act — another law to govern such misleading advertisements — were also being taken up by the health and AYUSH ministries.
It is in this context that the AYUSH ministry made its recommendation to ignore the rule.
Banned drugs
Fixed-dose combinations/ proposed ban, 2018
Rupali Mukherjee, August 24, 2018: The Times of India
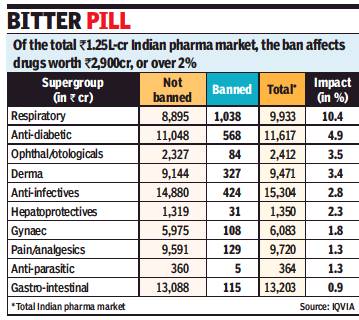
From: Rupali Mukherjee, August 24, 2018: The Times of India
The proposed government ban on over 340 combination medicines (fixed-dose combinations, or FDCs) will impact over 2% — nearly Rs 2,900 crore — of the organised retail market, with popular cough syrups, painkillers and flu medicines — like Phensedyl, Saridon, Sumocold, and Vicks Action 500 — facing action. A final decision on the ban is expected soon by health ministry, even as an expert panel — the Drug Technical Advisory Board, or DTAB — earlier recommended these “irrational and “harmful” medicines should be taken off the shelves.
An FDC contains two or more drugs combined in a fixed ratio, and made available in a single dosage form. They comprise around 50% of the Rs 1.25-lakh-crore market (moving annual total, or MAT, June 2018), with only certain combinations being marketed “irrational” and “harmful”.
Last year, the Supreme Court (SC) had asked the DTAB to review the matter and recommend which FDCs should be regulated or banned, following a protracted legal battle between the government and drug companies over the issue.
The banned combinations on the proposed list belong to several therapeutic areas like cough and cold syrups, gastrointestinal and anti-infective formulations, and dermatological medicines. The respiratory portfolio will bear the highest impact of 10%, followed by antidiabetic and anti-infective drugs, data culled from IQVIA, a leading technology-driven healthcare service provider said.
Gastro-intestinal medicine Panderm+ (Macleods Pharma) is the most impacted brand at 8% or Rs 233 crore in value terms in the banned category, followed by cough preparation Phensedyl Cough (Abbott).
IQVIA general manager South Asia Amit Mookim said, “The popularity of combination drugs has been due to the convenience of reduced pill burden at an affordable cost. After the ban implementation, the doctors may now have to change their Rx (prescription) habit and prescribe multiple plain drugs, instead of writing one FDC, which may have a huge cost impact on the patients. Further, retail pharmacists dispense medicines based on doctors’ prescriptions that mention the brand names. The banned FDCs are sold under multiple brand names, and looking into the combination of each such brand is an extremely complex process. Each pharmacy may have hundreds of brands with the same combination, and it will be difficult to identify the banned FDCs — which are given in pharmacological names — and eventually may pose a risk to the patients. Therefore, the industry will have to educate and create awareness among pharmacists for effective and safe implementation of the ban.”
Govt bans Saridon, 327 other combination drugs
September 13, 2018: The Times of India
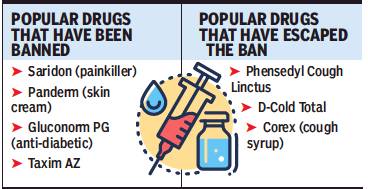
From: September 13, 2018: The Times of India
Among the roughly 6,000 brands estimated to be affected by the ban are popular drugs like the painkiller Saridon, the skin cream Panderm, combination diabetes drug Gluconorm PG, antibiotic Lupidiclox and antibacterial Taxim AZ.
The government had banned 344 FDCs on March 10, 2016 and later added five more to this list. However, manufacturers of these drugs contested the ban in various high courts and the Supreme Court. The SC on December 15, 2017 asked for the matter to be examined by the Drugs Technical Advisory Board. DTAB concluded that there was no therapeutic justification for the ingredients in 328 FDCs and that these could be a risk to people. The board recommended banning them.
In the case of six other FDCs, the board recommended restricted manufacture and sale subject to certain conditions based on their therapeutic justification. The SC ruled that the government could not use the DTAB report to prohibit 15 of the 344 drugs in the original list as these have been manufactured in India since before 1988. This exception covered several popular cough syrups, painkillers and cold medication with sales amounting to over Rs 740 crore annually. However, the court told the ministry that it could still look into the safety of these 15 drugs by initiating a fresh investigation if it wanted to ban them.
The All India Drug Action Network, one of the petitioners in the SC case, welcomed the ban and sought swift action on the 15 excluded FDCs.
‘Unsafe FDCs make up 25% of drug market’
The banned FDCs account for about Rs 2,500 crore and represent only the tip of the iceberg. In our estimate, the market for unsafe, problematic FDCs in India is at least onefourth of the total pharma market which is valued at Rs 1.3 trillion,” the All India Drug Action Network, a civil society group working on safety and access to medicines which was one of the petitioners in the Supreme Court case, said in a statement. It also sought a review of all FDCs in the market in the interest of patient safety as recommended by the Kokate Committee, constituted by the health ministry to examine FDCs.
Meanwhile, many large drug companies have claimed that over the last couple of years they have either phased out such drugs or changed the combination. The FDCs in question are less than 2%, they claim.
SC stays drug ban, allows Saridon, Piriton, Dart
SC stays drug ban, allows manufacture, sale of 3 FDCs, September 18, 2018: The Times of India
The Supreme Court allowed sale of Saridon, Piriton Expectorant and Dart — three fixed dose combination (FDCs) drugs — recently banned by the Centre along with 325 other drugs and permitted pharmaceutical companies to manufacture and sell the medicines.
A bench ofJustices R F Nariman and Indu Malhotra agreed to hear pleas from pharma majors challenging the Centre’s decision to ban the drugs.
The government took the decision on the recommendation of a panel constituted by the Centre on the SC’s direction to review safety, efficacy and therapeutic justification of FDCs. Hearing petitions challenging the Centre’s 2015 ban on some FDCs, the court in December referred the issue to the Drugs Technical Advisory Board (DTAB) for a fresh view before recommending action. FDCs are single dosage combinations of two or more drugs.
The review was ordered after the Centre challenged the Delhi high court order quashing its March 10, 2016, notification banning 344 FDC drugs citing health risks and lack of therapeutic justification. The ban on FDC drugs was enforced following a report by a six-member committee, which in January 2015 termed 963 FDCs as posing health threats. The court, however, said its Monday order will be applicable to FDCs given licence prior to 1988.
Challenging the Centre’s decision, manufacturing companies filed an application before the court saying contended the three FDCs were given licence before 1988 and the panel was not allowed to examine them.
Three drug manufacturers challenged the ban saying it was illegal and arbitrary, arguing their FDCs received licences before 1988 and the central panel was not allowed to examine them
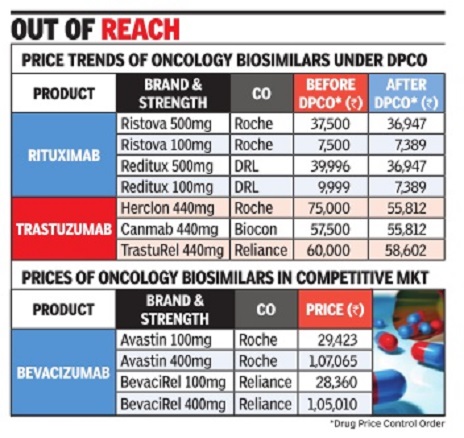
Biosimilars
2018: India no.1 in world
Rupali Mukherjee, Desi pharma cos top in biosimilars globally, October 29, 2018: The Times of India
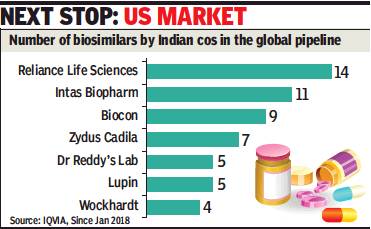
From: Rupali Mukherjee, Desi pharma cos top in biosimilars globally, October 29, 2018: The Times of India
India is leading the global biosimilar pipeline, with the largest number of products launched in the country and emerging economies. Biosimilars, developed by innovators, are approved versions of biopharmaceuticals — complex medicines manufactured using live organisms, as against conventional drugs that are based on chemical composition.
At present, domestic companies are marketing their products only in emerging markets, but the largest and most lucrative market for all pharmaceuticals — the US — is the next stop. The development not only indicates that Indian pharma companies are moving up the value chain after decades of making copycat medicines, it has, more importantly, led to treatment costs getting slashed by 20-40% in chronic and life-threatening illnesses, like rheumatoid arthritis and cancers.
“Reliance Life Sciences has pursued a strategy of developing, in an integrated manner, a wide array of biosimilars at competitive prices to meet patient needs on a global basis. Being recognised as the top biosimilar player globally, in terms of number of products, is one testimony of this strategy in practice,” says company president and CEO K V S Subramaniam.
Significantly, the entry of biosimilars has helped bring down prices for patients. To illustrate, net prices to innovators have come down 18% from Rs 22,000 for bevacizumab (a 100mg vial), 45% to Rs 3,000 for interferon beta (30-microgram pre-filled syringe), and 14% to Rs 19,000 for rituximab (500mg vial). Indian companies are exporting biosimilars to developing countries, primarily South Asia, Southeast Asia, Africa and Latin America.
Intas VC and MD Binish Chudgar said, “The estimate of the global biosimilar market is $9.5-13 billion by 2022, despite the fact that over $90 billion worth of biotech-based medicines are expected to go off-patent in the next few years. Much of the activity in biosimilars is taking place in Europe, while the US, which is the largest pharmaceutical market in the world by a wide margin, has not really opened up to these products. On the domestic front, with a very supportive regulatory authority, biotechnological medicines are set to become an important part of future healthcare landscape.”
PwC India leader (pharmaceutical & life sciences) Sujay Shetty said, “There are emerging signs that partnerships (Biocon with Mylan) are beginning to bear fruit. However, their success in regulated markets will depend on skills of each JV partner. India can dominate rest of world. Going forward, there could be competition from Korean and Chinese companies in the emerging markets.”
Chemists
Regulations/ 2018-19
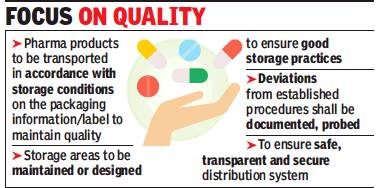
From: Rupali Mukherjee, Chemists may face legal action for violation of storage norms, November 25, 2018: The Times of India
Govt Plans Strict Guidelines To Prevent Drug Adulteration
Consumers can now expect to get better bang for the pills they pop. The Centre is planning to introduce stringent guidelines on distribution practices for drug companies. The regulations — expected once the health ministry’s expert panel, Drugs Technical Advisory Board (DTAB), approves them this week — will prevent widelyprescribed antibiotics and pain-relievers, insulins and vaccines from getting adulterated or contaminated during transportation and distribution.
Once in force, stockists and chemists who fail to comply with the specified storage and distribution norms will face legal action. At present, there are standards laid down for manufacturing, but have been practically non-existent for storage, transportation and distribution once medicines leave the company’s plant — giving rise to contamination and sub-standard medication in certain cases. There have been instances when popular medicines, including pain-killers, vitamins, antibiotics, anti-allergics have disintegrated or were sub-standard, and insulins and vaccines did not have the desired therapeutic effect, or were not effective.
Such issues arise if certain medicines, are not transported from the manufacturer to pharmacy through a cold chain, where essentially temperature is controlled (2°C to 8°C), or these are stored without refrigeration.
“The practices will ensure quality of medicines and integrity of supply chain, while preventing entry of any spurious drugs into the distribution channel,” sources told TOI, adding these will be taken up at the DTAB meeting on November 29.
The guidelines are to ensure quality and identity of pharmaceutical products during all aspects of the distribution process from the manufacturing plant to medical stores. These include procurement, purchasing, storage, transportation, and documentation. These regulations will be applicable on all outlets involved in any aspect of the storage and distribution of pharmaceutical products from the premises of manufacturer to the person dispensing or providing pharmaceutical products directly to a patient, the draft says.
Transportation of drugs is carried out by third parties in most cases. Involvement of unauthorised entities in distribution chain is also a concern.
Clinical trials
Rules eased/ 2016
The Times of India, Aug 04 2016
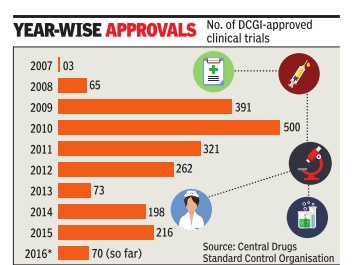
Sushmi Dey Clinical trials may rise as drug regulator eases rules The federal drug regulator has eased norms related to pharmaceutical research, a move that may boost the number of clinical trials but could also raise concerns on patient safety .
Drugs Controller General of India (DCGI) in a notification, said an investigator or researcher can undertake as many trials as approved by the ethics committee instead of the present cap of three. The regulator has also relaxed norms for hospitals or clinical sites undertaking such trials. Through a separate notification, the DCGI has revised the rule that prohibited any hospital with less than 50 beds to take up a trial.
Under the revised norms, the ethics committee has been empowered to decide whether asite is suitable for a trial irrespective of its bed capacity . It suggested the site should have “emergency rescue and care arrangements“.
The ethics committee is a panel of experts which examines the proposal of a pharmaceutical company or a researcher to conduct clinical trials or human experiments for new medicines.
Following random clinical trials and increasing number of deaths during such experiments, the government had earlier imposed a restriction of not more than three clinical trials to be conducted by an investigator.
The number of clinical trials in India went up rapidly around 2008-09. According to data available from the Central Drugs Standard Control Organisation (CDSCO), aro und 65 trials were approved in 2008, whereas it jumped to 391 in 2009 and 500 in 2010. However, following the Supreme Court's intervention, the approvals dropped drastically from 2011onwards.
The changes have cheered the industry . “Ultimately what this translates into is qualitatively better clinical trials as decisions will be guided by which investigator or site is best suited for a particular trial,“ Indian Society of Clinical Research president Suneela Thatte said.
Criticisms of/ allegations against Indian drugs
India says US report is an attack on cheap generic drugs/ 2019
Sushmi Dey, April 28, 2019: The Times of India
India trashes US report, calls it attack on cheap generic drugs
Govt: India Is Pharmacy Of The World
New Delhi:
India has outrightly rejected allegations in a US report about the country being a chief source of counterfeit medicines to the world and said it is an attack on low cost generic drugs — crucial to make healthcare affordable.
The ‘Special 301 Report’ by United States Trade Representative (USTR) slammed India and China as leading sources of counterfeit medicines distributed globally with 20% of all pharmaceutical products sold in the Indian market estimated to be counterfeits. “We strongly disagree with the observations made by USTR. We do not know the genesis and methodology of their findings. Instead, we view this as opposition to low cost generics and the thriving Indian drug manufacturing industry which is the ‘Pharmacy of the world’,” health secretary Preeti Sudan told TOI.
The USTR report, an annual review of the state of IP protection and enforcement in US trading partners around the world, has again put India on the ‘priority watch list’ for violation of intellectual property rights.
“In particular, China and India are reportedly leading sources of counterfeit medicines distributed globally. While it may not be possible to determine an exact figure, studies have suggested that up to 20% of drugs sold in the Indian market are counterfeit and could represent a serious threat to patient health and safety,” the report said. It also claimed that India exports counterfeit drugs to Africa, Canada, the Caribbean, the EU, South America, and the US. However, emphasising that generic drugs are low cost but quality products, Sudan said only certified pharmaceutical products are exported from here. Locally, over 75% of sales come from generic medicines.
Not surprisingly, India is named in the report for the country’s patentability criteria, compulsory licensing criteria and absence of an additional intellectual property monopoly-data exclusivity. “At a time when medicine prices are soaring... the report undermines the efforts... seeking to make medicines more affordable domestically. USTR’s push for more protection and enforcement of IP policies would keep medicine prices high globally and place lifesaving treatments out of reach for longer in developing countries,” says MSF which advocates access to medicines.
The report also said inadequate protection for trade secrets in a number of countries, notably China and India, puts US trade secrets at risk.
Drugs and pharmaceuticals made in India
Curbs by four EU nations
December 07 2014
Four European countries decided to suspend marketing authorization of 25 drugs, which had undergone tests at the GVK Biosciences facility European Medicines Agency (EMA) did say in a statement that it is reviewing the findings of non-compliance with good clinical practice at the GVK facility and determining its impact on medicines authorized on the basis of studies performed there. Germany , France, Luxembourg and Belgium have already decided to suspend marketing authorizations of these drugs. EMA's Committee for Medicinal Products for Human Use (CHMP) is now identifying, together with member states of the EU, the medicines covered by the inspection findings. French drug regulator ANSM has said on its website that Belgium, Germany , Luxembourg and France decided to suspend the marketing authorizations for the medicinal products concerned. “Although these documents are not essential to the demonstration of bioequivalence, the ANSM decided, as a precaution, to suspend the marketing authorization of 25 marketed generic drugs.
Drugs and pharmaceuticals sold in India
2008- 2014: Drug launches reduced by 80%
Dec 27 2014
New drug launches drop 80% in 6 yrs
Sushmi Dey
Launches of new medicines in India have come down by nearly 80% during 2008-2014 and drug manufacturers blame price regulations and policy uncertainty for making India an unattractive destination for both domestic as well as multinational pharmaceutical companies.
In 2008, 270 new drugs were approved for sale in India, whereas it dropped to 44 and 35 in 2012 and 2013, respectively. In 2014, only 56 new medicines were approved till November, government data shows.
“Most of the big pharmaceutical companies have knocked out India from their list of key markets. The reason is stricter policy measures cutting down on margins, making it less attractive as a business proposition for future growth,” a senior executive in a leading domestic pharmaceutical company said.
A stricter regulatory regime, which not only brings down drug MRPs but also continuously expands span of price control, is cited as the main reason why drug manufacturers are losing their India focus. Industry of ficials also blames looming uncertainty in policy as another reason for companies to delay product launches. Apart from pricing, the pharma industry has been facing hiccups in foreign investment, new drug approvals, as well as clinical trials.
Government and regulators, however, brush aside such concerns. According to a senior official in the National Pharmaceutical Pricing Authority (NPPA), the price regulations are as per the policy and MRPs are fixed based on average price of medicines in that segment. “There is no reason for a particular company to find it unviable when others are making the same drug at a lower price,“ the official said, adding that market surveys show a huge disparity in pric es of similar medicines sold under different brands.
The number of drug approvals peaked in 2008, when as many as 270 new drugs were granted approval, followed by 217 in 2009, 224 in 2010, and 140 in 2011, the data shows.
However, since 2012, when the government released the National Pharmaceutical Pricing Policy bringing in 348 medicine formulations un der price control, the new drug launches started reducing drastically. “The role of NPPA is to implement the policy in letter and spirit and not create confusion leading to instability in the drug industry,“ another senior industry official said.
Many medicines were also found missing from the market following stringent regulatory measures.
2018: domestic retail grows 10%
Rupali Mukherjee, January 16, 2019: The Times of India
From: Rupali Mukherjee, January 16, 2019: The Times of India
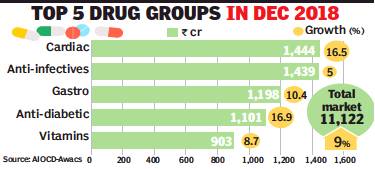
The domestic pharma retail market registered a robust growth rate of around 10% in 2018, nearly doubling year-on-year, buoyed by higher volumes and launch of new drugs. In 2017, the market was impacted by the introduction of GST, resulting in a meagre growth rate of 5.5% — the lowest in recent years.
Anti-diabetics, cardio-vascular, respiratory and derma medicines ended the year with strong double-digit growth, while overall drivers of the Rs 1.29-lakh-crore market include higher volumes (4.8%), price increase (2.2%) and new launches (2.4%), data from market research firm AIOCDAwacs said. For December alone, the market showed 9.8% growth, higher than in November. In fact, growth has been consistently above 9% over the last four quarters with Abbott, Lupin, Torrent and Intas growing at strong double-digit rates during the year, it said.
Anti-diabetic therapy human premix insulin Mixtard topped the pecking order, followed by anti-diabetes medicines including Glycomet GP, Lantus and Janumet for the year. Ayurvedic hepatic protector Liv 52 assumed the fifth slot. Anti-infectives is the largest therapy, followed by cardiac, gastro-intestinal and anti-diabetic. Expansion was driven by top domestic companies contributing close to 43%, and collectively outgrowing the market. The north and west zones in the country registered strong growth in double digits.
Glenmark Pharma president (India formulations, Middle East and Africa) Sujesh Vasudevan said, “The domestic pharmaceutical market saw good growth in 2018, driven by strong performance of anti-diabetic, cardiology and dermatology segments. In the current year, we expect doubledigit growth for the domestic pharma industry as companies continue to improve productivity and brand-building”.
MNCs grew 9% during December, while domestic companies recorded higher growth at 10%. Among the top MNCs, Boehringer Ingelheim was the fastest growing, followed by Bayer and Astra Zeneca. While anti-infectives grew 5%, respiratory segment slowed down at 2.7% during the month. As against this, dermatology grew faster at 10.5%, gastro-intestinal drugs posted double-digit sales growth (10.4%), while vitamins grew at 8.7%. Among the chronic therapies, anti-diabetic and cardio drugs posted robust sales growth at 16.9% and 16.5% respectively.
Sales of fixed-dose formulations, many of which have been banned and are facing regulatory glare, slumped, while the non-FDC (fixed dose combination) market grew 9.3% during December.
Types of medicines sold: 2018-19
Sushmi Dey , Oct 22, 2019: The Times of India

From: Sushmi Dey , Oct 22, 2019: The Times of India
Anti-infectives along with medicines for cardiac diseases, diabetes, gastro-intestinal and respiratory problems garnered the highest sales in the Indian pharmaceutical market in last one year, pointing to a rapidly growing burden of lifestyle-related ailments.
Indians spent over Rs 1,36,000 crore on medicines in last one year ending September 2019, of which 31% was spent on medicines for diabetes, heart disorders and other chronic ailments.
This is also the fastest growing segment at 13% as compared to acute therapies which accounted for 47% of the market but grew by 11.7% and sub-chronic segment at 10.7%, according to data released by All India Organisation of Chemists & Druggists (AIOCD).
While anti-infectives topped the list of medicines with highest sales value, those for cardiac problems, diabetes and gastro-intestinal disorders were among the top five, with only cardiac and anti-diabetes drugs registering double digit growth of 12.6% and 14.9%, respectively, for the year. The overall drug sales grew by 9.8% during the period.
People spent over Rs 17,000 crore on cardiac drugs, around Rs 15,400 crore for gastro-intestinal disorders and over Rs 13,360 crore on anti-diabetes medicines in the last 12 months. Anti-infectives accounted for the highest sales of over Rs 18,413 crore.
“Sales of anti-diabetic drugs has increased due to multiple factors like increased number of patients, more frequent diagnosis, increasing use of more expensive drugs and insulin. Importantly, use of conventional low cost drugs/insulin could help manage about 60-70% cases of diabetes in India, thus reducing out of pocket cost for patients,” says Dr Anoop Misra, Chairman Fortis C-Doc.
Maximum drug launches were in cardiovascular and respiratory segments. Around 78 new drugs were launched in September alone. Of these, 18 were to treat cardiovascular diseases, 17 were vitamins, minerals and nutrients and 14 were in in the respiratory segment.
Not just in terms of value but volume growth have also gained momentum, a senior industry executive said.
“Increasing disease burden, growing awareness, availability and affordability of treatment along with enhanced insurance penetration have all resulted in higher sales of medicines. Besides, there is no doubt that the NCD (non-communicable disease) segment is growing rapidly leading to higher growth in segments like cardiac and diabetes,” he said. The data also show the top ten corporates gradually increasing there market share in the chronic segment.
2022: The most popular drugs
DurgeshNandan Jha, June 17, 2023: The Times of India
From: DurgeshNandan Jha, June 17, 2023: The Times of India
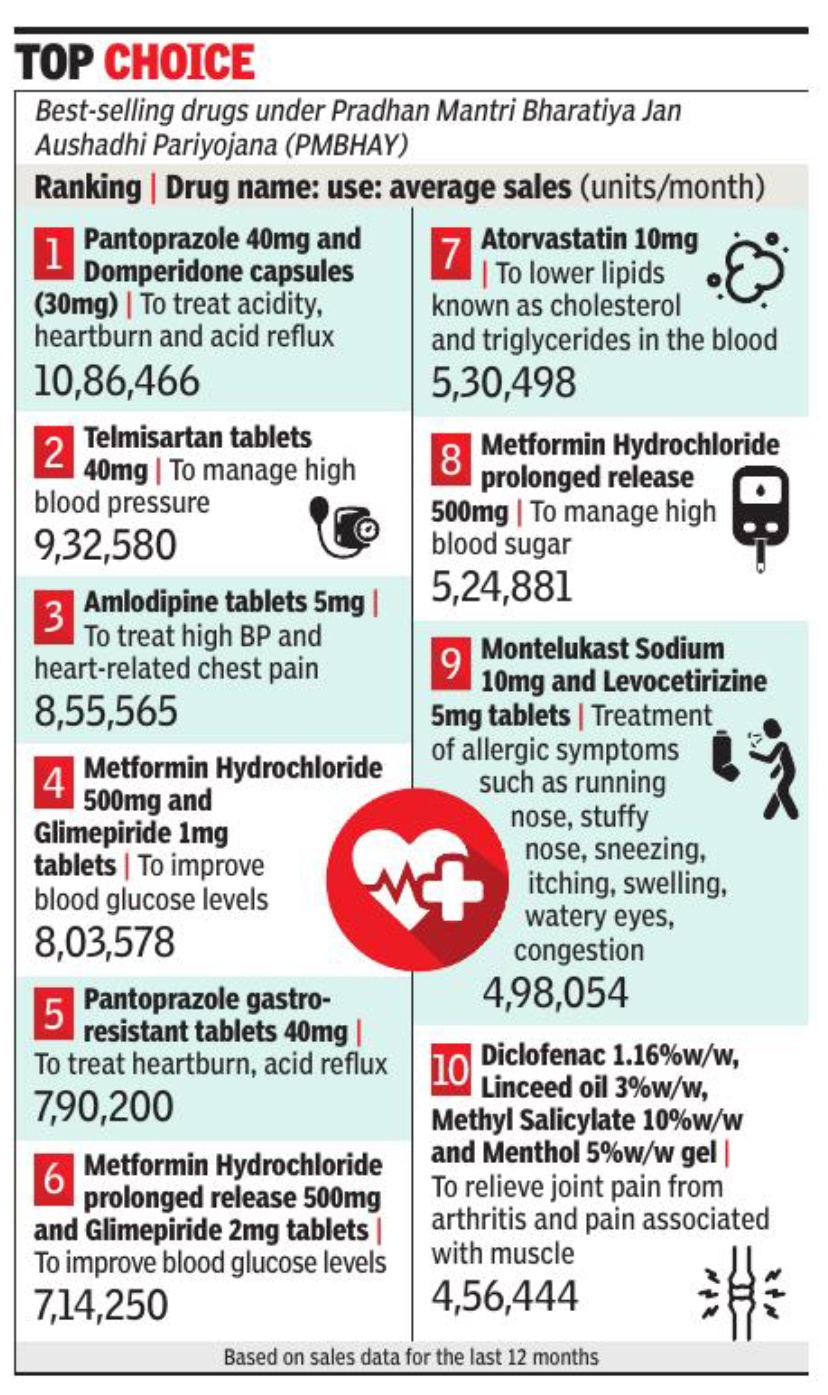
New Delhi : Pantoprazole (40 mg) and Domperidone (30 mg) capsules — a fixed-dose combination drug used for treating acidity-related problems — has emerged as the top selling product at Jan Aushadhi Kendras across the country.
India has 9,484 Jan Aushadhi Kendras which sell generic drugs at affordable prices to the public under the Pradhan Mantri Jan Aushadhi Pariyojana (PMBJP).
According to sales data of these stores for the last 12 months, an average of 10. 8 lakh units (each unit contains 10 capsules) of Pantoprazole (40 mg) and Domperidone (30 mg) capsules were sold at these stores on a monthly basis, the highest of all products sold by them. This was followed by Telmisartan (9. 3 lakh units monthly sales) and Amlodipine (8. 5 lakh units monthly sales), which are used to treat high blood pressure.
Ravi Dadhich, CEO, Pharmaceuticals and Medical Devices Bureau of India (PMBI), told TOIthat the generic drugs sold at the Jan Aushadhi Kendras are 50%-90% cheaper than the branded alternatives, which is why more and more people are opting to buy them. “Of the top 10 best-selling products at the Jan Aushadhi Kendras, 30% are products that are used to treat high blood pressure and diabetes. These are chronic conditions and treatment involves longterm medication. By opting for generic drugs, people can save a lot of money spent on drug purchase,” he said. The product basket of PMBJP comprises over 1800 drugs and 285 surgical equipment. Dadhich said that to ensure quality of products sold at the stores, all drugs are procured from World Health Organisation – Good Manufacturing Practices (WHO-GMP) certified suppliers. “Each batch of drug after its receipt at the warehouses is tested at laboratories accredited by ‘National Accreditation Board for Testing and Calibration Laboratories’ (NABL) for ensuring best quality,” the PMBI CEO said.
The major therapeuticgroup of medicines availableat the stores are: antibioticsand anti-infectives; cancerdrugs; cardiovascular drugs;and vitamins and mineralsamong others.
In 2019, thegovernment also startedselling sanitary napkins atRs 1 per pad. In 2014-15, there were only80 Jan Aushadhi Kendras andtheir sales value stood ataround Rs 7. 29 crore.
Earnings, profits
2012-18; exports to USA in 2018

From: Rupali Mukherjee, US-focused pharma cos see 30% drop in FY18 earnings, June 15, 2018: The Times of India
Regulatory clampdowns, increased competition and channel consolidation in the US have led to a drop of 30% — one of the worst — in earnings of USfocused Indian pharma companies in FY18. But the good news is there are signs of recovery with a strong doubledigit growth expected in FY19.
Analysts expect net profit of these companies to grow nearly 23% in fiscal 2019, fuelled by a rich and mature pipeline, a ramp-up in capacity, and a favourable base (GST and US tax impact).
Moreover, US revenues for the top 10 US-focused companies — Sun Pharma, Dr Reddy’s, Cipla, Lupin, Aurobindo, Glenmark, Cadila, Alkem, Torrent and Alembic — are expected to rebound, and grow in single digits in FY19, after the huge drop of nearly 12% witnessed in FY18.
Analysts believe fiscal 2019 may see a gradual comeback for large-cap pharma companies, driven by regulatory resolutions, and a moderating price erosion. Recently, beleaguered Sun Pharma resolved its regulatory issues at its Halol plant after a gap of over two years.
Most Indian pharma cos have scaled up US ops
Most companies were forced to go slow in US FDA (Food and Drug Administration) filings, as they were bogged down with their key plants being under the regulatory scanner. However, over last two years, companies have invested heavily to expand their capabilities — both in manufacturing and research and development.
Three years ago, a majority of US revenues of most Indian pharma majors was contributed by two plants. Since then, they have scaled up and diversified filings across multiple facilities. This has significantly reduced concentration risk with respect to future regulatory adversity, said an analyst from HDFC Securities in a note.
Glenmark Pharmaceuticals CMD Glenn Saldanha said, “We have had a good start to the new financial year with two good approvals, especially the approval of generic Welchol.
Though price erosion still remains a challenge in the US generic market, new product approvals will be key to growing the US business. We hope that we will be able to launch 10 to 15 new products in this year, which will augur well for our US generic business.”
For most companies, several product launches across generic and specialty categories are expected in the second half of FY19. With a rich pipeline, fiscal 2019 could be a breakthrough year in terms of niche product opportunities, the note says, including Sun Pharma’s (Tildra, Sceiera, Yonsa), Lupin (Solosec, Levothyroxine, generic Ranexa), and Dr Reddy’s (generic NuvaRing, generic Suboxone). Additionally, inhalers, bio-similars, trans-dermals and niche injectables are expected to be launched from FY20. A major reason for earnings having bottomed out is price erosion moderating in the US, experts say. Price erosion has been ever-present in the generics business. However, it has historically been in the range of 5-6%, while over 2016-18, there was a double-digit decline.
Most players have witnessed moderating price erosion during the fourth quarter FY18, and expect a stable singledigit price decline ahead.
2018-19

From: Sushmi Dey, April 28, 2019: The Times of India
See graphic:
Pharma exports rose 11% in FY19
Exports
Medical consumables: 2022-23
DurgeshNandan Jha, May 8, 2024: The Times of India
New Delhi: India, for the first time, has become a net exporter of medical consumables and disposables, reversing the earlier trend where overseas shipments of products such as needles and catheters completely dominated the market.
The country exported consumables and disposables worth $1.6 billion, while it imported products worth only about $1.1 billion in 2022-23, Union pharma secretary Arunish Chawla said. Exports went up 16% compared with the previous fiscal, while imports fell 33%.
Govt is seeking to replicate this success in other segments too, such as surgical instruments and electronic equipment, so that there is lower dependence on imports, Chawla said on the sidelines of an event by his department & CII.
Govt’s push for reducing import dependence for key pharma products and devices got a major thrust postCovid outbreak after China controlled supplies of everything from basic chemicals to PPEs and testing kits. India is known as pharmacy of the world because of its generic medicines and low-cost vaccines. In the medical devices sector, how- ever, the country remains heavily dependent on imports with nearly 70% of the products being sourced from other countries. China is among the major sources of imports.
Centre has further divided medical device sector into segments such as cancer therapy, imaging, critical care, assistive medical devices, body implants, surgical instruments and hospital equipment, consumables & disposables, and IV Dinstruments and reagents. The pharma secretary said deliberations are on to identify important medical devices from each segment, assess their import-export dynamics, examine duty structures, and their implications across the value chain.
“During Covid, demand for consumables and disposables increased tremendously which pushed the industry towards augmenting its manufacturing,” said Himanshu Baid, chairman of CII’s national medical technology forum.
Fixed dose combination (FDC) medicines
Health ministry bans 344 FDC drugs, HC lifts ban/ 2016
Sushmi Dey, Dec 5, 2016: The Times of India
`344 FDC drugs limit therapy choices'
Public health groups have expressed concerns over the Delhi high court order lifting the ban on 344 fixed dose combination (FDC) medicines, imposed by the government.
“This is a huge setback to efforts aimed at bringing a semblance of order into the absolute anarchy that exists in India's pharmaceutical market,“ said a statement from Jan Swasthya Abhiyan.
The health ministry had banned 344 FDC drugs terming them as irrational and raising concerns over their misuse. These included some of the popular pharmaceutical brands such as Pfizer's Corex and Abbott's Phensedyl. However, recently the Delhi HC overturned the government's ban providing a major relief to the pharmaceutical companies.
Public health and advocacy groups have asked the government to appeal against the HC order as it has the potential to impact healthcare cost as well as quality of medicines. Health groups said lifting of the ban seems to be predicated on “perceived procedural issues“ which fundamentally abrogate the right to life and healthcare.
Jan Swasthya Abhiyan said use of FDCs increase cost of medication, exposes populations to a larger array of adverse effects and limits the choice of therapy as they may combine drugs with different dosage schedules. Further, some of the cough syrups in the ban order are primarily being used as addictive substances and not as therapeutic agents.
FDCs include two or more active pharmaceutical ingredients combined in a single dosage form.
“The Delhi High Court order does not appear to address the issues of rationality of the FDCs and the resultant adverse effect on public health,“ it said, asking the government to plug legal and regulatory loopholes so that the ban order can be restored.
“It is of utmost importance that the government, as a custodian of public health, act decisively to de fend it and strengthen regulatory mechanisms,“ JSA said.
The HC verdict has come as a major set back to the government which was planning to ban more FDCs to eliminate irrational combination drugs causing anti-microbial resistance. In some cases the toxicity is so high that it can even lead to failure of organs, officials say. There are also concerns many of these FDCs being available over-the-counter without doctors' prescription, which is leading to their misuse.
While many of these products are sold at the chemists level, officials and health experts say often adverse events are not reported because patients do not come back to doctors unless these drugs are used repetitively , leading to severe problems.
Estimates show around FDCs comprise over 40% of the total Rs 1 lakh crore annual Indian pharmaceutical market. However, only around 16 FDCs are part of the National List of Essential Medicines.
Foreign countries’ approvals to sell Indian drugs
2018-19: USFDA approvals increase 9%
Rupali Mukherjee, May 6, 2019: The Times of India

From: Rupali Mukherjee, May 6, 2019: The Times of India
Domestic phar ma companies received 372 approvals to launch generic drugs in the US in fiscal 2019, up 8.6% from 340 in the previous year. The development comes even as India got 15 warning letters in calendar 2018, lower than the US with 19, and China which topped the list with 24 warning letters. As against this, India had the ignominious distinction of topping the list with 9 warning letters in 2015, and has since appeared to have cleaned up its act.
In terms of approvals, Zydus Cadila topped the list with 60, with Indian companies cornering nearly 40% volume share in the highly lucrative $60-billion-plus US generic market — a key driver of growth for the domestic industry. In an indication of having resolved data integrity issues by investing in skill sets, domestic companies grabbed the opportunity by the US Food and Drug Administration (USFDA) to speed up generic competition.
Indian generic filings have been rising year-on-year, unfazed by regulatory pressure from the USFDA, and a spate of warning letters issued to their facilities over the last couple of years. Over 2015-17, Indian companies faced intense regulatory glare from the US, with nearly all top companies having been issued warning letters over manufacturing violations at their plants. This seems to have changed last year with fewer warning letters for Indian companies, compared to other major countries.
Also, USFDA’s erstwhile chief Scott Gottlieb had last year announced a slew of policy measures to strengthen and streamline the generic drug-review process, setting record numbers for generic drug approvals.
Imports from China
Active pharmaceutical ingredients
2016-19

From: Sushmi Dey, June 23, 2020: The Times of India
See graphic:
The import of Active pharmaceutical ingredients (APIs) from China and other countries, 2016-19.
Import of ingredients
2017: 26% dip in import from China
Chethan Kumar, 26% dip in drug ingredient import from China, January 22, 2018: The Times of India
From: Chethan Kumar, 26% dip in drug ingredient import from China, January 22, 2018: The Times of India
See graphic:
APIs imported from China to India, 2015-17
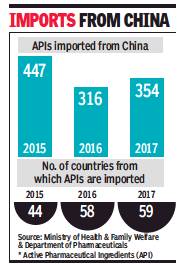
In 2017, India imported 354 Active Pharmaceutical Ingredients (APIs) from China, a 26% dip compared to 447 in 2015, largely achieved because the number of countries India imported such material from increased from 44 in 2015 to 58 in 2016 and 59 in 2017.
The number of APIs imported from China has grown slightly compared to 2016 (316) though. Also, import from China is still the highest in this category, accounting for 66% of all imports in 2017 — Rs 12,254.97 crore of the Rs 18,372.54 crore. The trend has remained similar in all the three years.
Many of these APIs go into 12 essential drugs listed by India — paracetamol, metformin, ranitidine, amoxicillin, ciprofloxacin, cefixime, acetylsalicylic acid, ascorbic acid, ofloxacin, ibuprofen, metronidazole and ampicillin — and 8 of which are also on WHO’s Model List of Essential Medicines.
Officials from the Department of Pharmaceuticals (DoP) and industry experts point out that the import of APIs is because of economic considerations, but experts also pointed out the issue of quality that comes with such over-dependence.
Earlier this month, India banned import of APIs from six Chinese firms claiming they did not meet the required quality standards.
Drugs Controller General of India Dr GN Singh said: “Our mandate is limited. We are concerned about the quality of imports and are always keeping a track of it. The ban on those firms is routine and should not been seen as targeting the Chinese companies.”
He said that India is also manufacturing APIs and there are more than 500 products, but most of them are being exported. Indian Pharmaceutical Association president Dr Rao VSV Valdlamudi said drug prices in the domestic market are controlled and while the APIs being exported command the price their quality demands, they do not fetch the same price domestically.
“The problem of over-dependence, however, is a much larger issue. If China stops importing from India, will India be able to turn all its exports to the domestic market? Over-dependence has to reduce. At present the government doesn’t take into consideration many variables and overheads in production and subsidies, as some argue, is not a longterm solution,” Rao said.
India has been putting in place some systems, but chemicals and fertilisers minister HN Ananth Kumar, while blaming legacy, concedes the Centre is not fully happy with the progress.
“Historically, there has been too much dependence on China and previous governments have done nothing about it. We took a twopronged approach.
First, from 0% customs duty on import of APIs from China, we made it 7.5% which is very good for domestic API makers. As per the Dr Katoch Committee report, we initiated having bulk drug parks and the first will come up near Hyderabad,” he said.
He said the Centre will provide up to Rs 500 crore for common facilities like power, water, effluent treatment, testing and trading facilities at such parks, which will reduce production cost by 30% making it globally competent, even compared to China.
The industry in India
Helping fight AIDS, Covid 19
SWAMINATHAN S ANKLESARIA AIYAR, January 3, 2021: The Times of India
Arguably, the most-hated industry in the world is the pharmaceutical industry. Since dying people will pay anything, the normal price resistance of consumers disappears. So, profit margins for new patented drugs can be humongous. When anti-AIDS drug cocktails were invented in the 1990s, US drug companies charged a whopping $15,000 per year. This created an uproar. To mollify critics, major US companies offered the drug “at cost” to poor countries — just $1,500, they said.
But Cipla, an Indian company, was already exporting the drugs at just $800. Cipla was lambasted by US companies as a “pirate” violating patents. Cipla retorted that the US companies were the real robbers and pirates. US President Bill Clinton ultimately sided with the activists.
Production shifted massively to countries like India, and prices kept falling. By 2010, the cost was just $200. No wonder activists denounced US drug companies as killers. Yet, ironically, these very companies had created the cures and saved millions.
The hated drug industry has just performed a miracle, producing several different vaccines against Covid in a few months. It had proved impossible to develop any vaccine at all for several viruses, including AIDS. When Covid struck, sober specialists noted that new vaccines took at least five years to be created, tested and approved. Bill Gates said we would be lucky to get an anti-Covid vaccine in 18 months. Yet in less than a year, vaccines galore have emerged. Russia and China were among the first to create and approve their own vaccines. Western experts cautioned that these countries had not followed all the usual safety protocols. However, it can make sense to shorten test procedures to expedite a vaccine that could save millions of lives.
Pfizer and Moderna in the US have produced vaccines following the usual protocols and are ready for mass vaccination. AstraZeneca and Oxford University have developed a different vaccine which — at the insistence of Oxford University — will be sold at just $3-4 per dose in poor countries. This vaccine can be stored at 2 to 7 degrees Celsius in ordinary refrigerators, making it suitable for poor countries like India lacking the super-cooling facilities required by the Pfizer and Moderna vaccines.
Maybe half the population of most countries will be vaccinated by late 2021, slashing further transmission of Covid. Gradually, people will resume travel, office meetings, social gatherings and tourism.
What lessons flow from this? First, the pharma industry is a saviour, not a killer. The international patent system is seriously flawed. To make up for huge R&D losses on drugs that fail tests, companies make enormous profits on the few that work. This can sound odious. But remember, the drug companies are the saviours that have doubled life expectancy in the last century, curing dozens of diseases once incurable, relieving the world of immense misery and death.
People have shown that they will happily give up their entire life savings for another year or two of life. By that standard, the life-extending services of drug companies make them among the greatest saviours in history.
What the Covid example shows is that government guarantees can make a huge difference. R&D is expensive. If governments are serious about health, they should offer significant funding for basic research, research on diseases of specific local interest, and for clinical trials.
For tackling tropical diseases, developing countries as well as aid consortia and institutions like the World Bank should guarantee to buy a large quantity of promising drugs even before expensive testing begins. This can be linked to price caps for drugs that clear testing.
In theory, all medical R&D could be done by governments and offered patent-free to all. However, the historical experience in this has been dismal. The Soviet Union and its Red empire stretching across Eastern Europe and Cuba boasted of good and free healthcare but failed to produce significant new drugs. Virtually all the hundreds of new medicines that saved millions of lives were created by profit-motivated R&D by drug companies. The social motivation of public sector research proved insufficient.
Drug companies have been found guilty of many sins: of cartelisation to raise prices or diminish competition; of fudging clinical trials; of promoting unsuitable or even bogus drugs; of bribing doctors to promote their particular medicines; of encouraging addictive opioids; and of enormous profits on some drugs. Yet the very same sinners have saved millions of lives through R&D. We must harness their skills while reducing the odium of super-profits on a few drugs.
Covid shows that we need new systems of government support for medical research. Public health is a public good that governments have a duty to improve. This does not mean just price controls and hospital subsidies but guarantees and funding for relevant R&D on diseases.
Influencing academies, research
Pharma industry influences Academy of Paediatrics
Rema Nagarajan, How pharma firms influence paediatricians' body, Mar 27, 2017: The Times of India
A Parliament question “regarding the influence of vaccine-makers on the immunisation plan“ has raised the issue of funding of the Indian Academy of Paediatrics (IAP), the body representing over 23,000 paediatricians in India. A look at its finances shows just how dependent the IAP is on funding from the pharma industry , especially vaccine manufacturers.
Most of the industry funding comes as sponsorship for IAP's annual conference, Pedicon, and funding for the association's activities under the Presidential Action Plan.
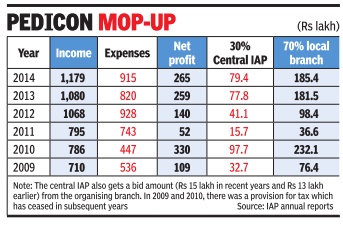
In 2016, IAP earned Rs 5.5 crore, of which almost 30% was combined income from Pedicon (55 lakh) and the Action Plan (Rs 1.1 crore). Pedicon's share is usually much larger, touching Rs 93 lakh and Rs 94 lakh in 2013 and 2014 respectively , including the bidding charge of Rs 15 lakh that the organising branch has to pay the central IAP (CIAP).
The growing dependence on industry was raised by IAP treasurer Pravin J Mehta in the 2013 annual report when he said: “Our expenditure per year is approximately 2.77 crores. Our fixed income... is approximately 1.77 crores.That means every year we are dependent on variable income of rupees one crore...Major chunk of this variable income is from Pedicon. This means that in unforeseen circumstances of Pedicon not doing well financially , it will be difficult to match income with rising expenditure.“
The “lead sponsor“ of Pedicon 2017 held in Bengaluru in January this year was Bharat Biotech, a leading vaccine company . According to the brochure, platinum sponsorship was Rs 5 crore. So was Bharat Biotech the platinum sponsor? Dr Govindaraj M, the chief organising chair man, claims he does not know.Other vaccine companies like Wyeth and Sanofi too got prominent mention at the venue, which could mean that they were diamond (Rs 4 crore), gold (Rs 3 crore) or silver (Rs 2 crore) sponsors. Judging by the sponsorship brochure, this conference could have raked in over Rs 15 crore.
Pedicon is supposed to be an academic conference, but the discussions regarding it seem to revolve primarily around the revenue it generates and sharing of the profits between the central IAP and the organising branch in a 30:70 ratio. The profits generated by some Pedicons have been over Rs 3 crore.
IAP's 2015 annual report talks about the escalating costs of the annual conference with per delegate costs rising from Rs 10,000 for four days to over Rs 20,000 in the past three years. Total expenses had more than doubled from about Rs 4.5 crore in 2010 to over Rs 9 crore by 2014.
The report noted that IAP had no mechanism for oversight of expenses and though the income from the conference has continued to rise, profits had remained almost static. There was a suggestion that the IAP should take complete charge of Pedicon finances. But that is being resisted.
In a critical note in the annual report, Dhananjay Shah, founder convenor of the first finance committee, wrote: “Pedicon organisers have been kindly making luxurious arrangements for the 50 odd group of newly elected EB members who are provided a five-star accommodation at a luxurious hotel for full five days during their Pedicon as an expense item of the Pedicon. The EB meeting is actually a CIAP (central IAP) function and has nothing to do with the Pedicon event.“
He also warned that the money available in IAP cof fers could be an “attractive bait for those with evil designs“ and that candidates were already spending sums of even Rs 50 lakh “to win the presidential crown“. IAP's academic role “will take a severe beating and thrashing“, he apprehended.
Gala cultural evenings and bar nights push up the cost of these so-called academic conferences. However these are not unique to IAP.Almost all doctors' associations organise these lavish sponsored conferences ostensibly to educate doctors. From the annual conference of national associations of specific specialties, to those organised by state or city branches, all are funded by pharma companies that shell out crores to oblige the doctors.
In 2014, the IAP alone had 27 state branches and 314 city district branches. The Cardiology Society of India has 26 state branches, not including the sub-specialty councils. Doctors insist they are never influenced by the generosity of these companies, but that begs the question of why for-profit companies would spend crores without expecting returns in the form of `prescription support' from the doctors.
Manufacturers
Ownership changes hands/ M&A
2010-23
Rupali Mukherjee, Oct 9, 2023: The Times of India
Mumbai: Multinationals’ drug portfolios are thinning. Consolidation is underway in the formulations business. Some Indian promoters are seeking an exit. These factors are heating up dealmaking activity in the domestic pharma sector.
Mergers and acquisitions in the pharma sector are expected to pick up as promoters of high leverage companies cash out amid rising compliance costs and pricing pressures in the domestic and the US market.
Indian strategic buyers are increasingly acquiring domestic assets, while private equity (PE) biggies like KKR, Caryle, and Advent are getting aggressive and building platforms in both high-growth domestic formulations and API businesses — as in the case of recent deals such as JB Chemicals and Suven Pharma. The PE activity that started just before the pandemic will accelerate over the next few years, industry experts say.
Over the years, promoters have been cashing out in the wake of increasing price control, higher regulatory scrutiny in the US, and the disappearing lucrative Para IV opportunities, leading to a tough business environment, said PwC India’s global health industries advisory leader Sujay Shetty.
Incidentally, some of these factors also triggered the sell-out of pharma biggie Ranbaxy by the Singh family in 2008. Ranbaxy's promoters, led by Malvinder Singh, decided to sell their entire family stake of nearly 35% to Japan’s Daiichi Sankyo for Rs 10,000 crore — one of the largest deals in Indian pharma.
Now, the promoters of Cipla — India’s third-largest drug firm — are planning to divest the family’s stake, with the second generation not keen to continue running the business. Recently, Mumbai-based Glenmark Pharma announced a divestment of 75% in its subsidiary, Glenmark Life Sciences, to Ahmedabad-based detergents-to-cement conglomerate Nirma for Rs 5,652 crore, and in another deal, US-based Viatris (erstwhile Mylan), is selling its active pharmaceutical ingredients and women’s healthcare businesses in India for a combined value of $1.2 billion (nearly Rs 10,000 crore), as part of a $3.6-billion global divestment. Further, large domestic players are doubling down on India as an attractive diversification from a US generics market beaten up heavily by price erosion. As a result, several deals were inked where Indian companies snapped up high-growth brands from local sellers at attractive valuations. For instance, last year, Mankind Pharma swooped on Panacea’s domestic formulations’ business while three years ago, Dr Reddy’s acquired some Wockardt brands, and Torrent Pharmaceuticals bought Unichem’s branded formulations assets in 2017 (see graphic).
As against this, a decade back, mainly foreign firms like Abbott, Daiichi Sankyo, Sanofi made headlines with sizeable buy-outs of domestic firms like Piramal Healthcare, Ranbaxy and Shantha Biotec, respectively.
“The pharma M&A landscape has changed over the last few years and has been mainly driven by how the markets (both domestic and international) have evolved competition, resource allocation and PE interest. On one hand, we have seen global Big Pharma pruning their branded generic portfolio to domestic companies, rationalising their portfolios by selling off their upstream businesses (read APIs, etc) and buying into mid-market brands (including prescription, OTC and consumer brands) to PE players doing buy-outs and roll-up plays across APIs, CDMOs and branded formulation,” said Sunil Thakur, partner and head of South Asia at Quadria Capital, a healthcare PE fund.
Medicines/ Pharmaceuticals that sold the most in...
2017-18
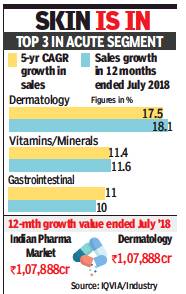
From: Rupali Mukherjee, In India, derma biz grows fastest in retail pharma, October 1, 2018: The Times of India
Skin-Related Issues Draw Most Concern
Indians are virtually turning the age-old adage that beauty is only skin deep on its head. Issues like cough or cold or even lifestyle problems such as diabetes and heart disease are not worrying us, but surprisingly, it’s skin-related ailments which are causing concern.
Dermatology is showing the fastest sales growth of 17.5% with a CAGR (compounded annual growth rate) of five years in the domestic pharma retail market. Interestingly, this is even higher than the sales growth witnessed in anti-diabetes (16.8%), or heartdisease pills (10.7%), over a CAGR of five years.
Increasing incidence of fungal infections, greater awareness of skin-related issues and skin care and a growing desire to look presentable are factors driving the growth, say experts.
Overall, the Indian pharmaceutical market (IPM) has witnessed a CAGR growth of 11.2% in last five years.
In the 12 months ended July 2018, IPM growth was 11.6% while its market is valued around Rs 1,07,888 crore. Dermatology, on the other hand, has witnessed CAGR growth of 18.1% in 12 months ended July 2018. The value of dermatology market is Rs 8636.3 crore.
Earlier, people just lived with a mole, white patches and pigmentation issues, or hair loss. Patients are more conscious of their appearance today and seek medical help for aesthetic problems, dermatologists told TOI. Nearly half of their practice now comprises of such cases, as against two to three patients earlier.
Sales within the derma category also mirror the trend, with skin-related medication driving the high growth. Overall, the largest category is anti-fungal topicals (43.8%) — medication applied on skin, followed by anti-fungal oral drugs (35.3%), data culled from healthcare service provider IQVIA said.
Sujesh Vasudevan, president India and MEA business of Glenmark Pharmaceuticals, said: “Prescription dermatology segment has witnessed strong growth in the antifungal space driven by general awareness among the population, and also due to pollution. Thus, most of the growth in the dermatology segment has been volume led. We continue to believe that that the dermatology segment will consistently grow at a rapid pace. This has also resulted in intense competition and new players constantly entering this segment”.
Industry experts point out that the huge growth in derma includes only those medication prescribed by doctors and not the exorbitant botox treatment done for cosmetic purposes to reduce facial wrinkles or fine lines.
For another industry player, GlaxoSmithKline Pharma, the topical antibiotic portfolio (used to cure infections caused by bacteria in wounds) is important. Managing director of GSK Pharma A Vaidheesh said, “Dermatology is one of the important focus segments for GSK India where we have a strong heritage and ambitious plans for patient access. We are focusing our efforts on key brands to drive growth in identified therapy areas where there is significant unmet patient need.”
However, indiscriminate use of harmful steroid-containing creams which are a combination of anti-fungal, anti-bacterial and anti-allergic, is a huge issue. “Use of unethical and unscientific combination creams (containing steroids) has risen substantially. They are increasingly prescribed by doctors (other than dermatologists), while a large percentage are bought over the counter, even though these should not be sold without a proper prescription,” said dermatologist Belinda Vaz.
Experts stated that over the last few years, sales of medicines for chronic ailments, like diabetes and hypertension, have seen strong growth in the Indian market while those in the acute segment have been stagnating.
2020-21
Rupali Mukherjee, May 11, 2021: The Times of India
Call it the Covid effect. Anti-viral drug Favipiravir, used for Covid-19 treatment, has rocketed to the rank of the largest-selling pharma brand in April for the first time ever in the domestic retail market, reports Rupali Mukherjee.
The drug, marketed by Mumbai-based Glenmark as Fabiflu, posted sales of Rs 352 crore in April alone, overtaking sales of drugs for lifestyle ailments such as diabetes, which have long dominated the market.
In April, Fabiflu overtook sales of other top-selling drugs being used in Covid-19 therapy, including health supplement Zincovit and pain-killer Dolo.
Early 2021
Fabiflu mops up ₹762cr sales in last 12 months
During the pandemic, the top pecking order witnessed a change with Zincovit becoming the largestselling pharma brand in October last year, while others like Monocef, Dolo and Betadine grew at a strong clip this year.
For the 12-month period ended April, Fabiflu mopped up Rs 762 crore sales, higher than anti-diabetic drug, Glycomet-GP’s Rs 564 crore, with around half coming in April alone, according to data culled from pharma research firm, AIOCD-Awacs.
In September, Glenmark touched its highest monthly sales of around Rs 60 crore, and thereafter tapered off, in line with the decrease in Covid-19 caseload. Sales witnessed an upswing post mid-March as cases started rising, while in February, there were insignificant sales.
“Our every effort from the beginning has been to provide doctors a safe and effective treatment option in an out-patient setting and reduce the burden on the country’s hospitals and healthcare system'’, Glenn Saldanha, chairman and MD Glenmark Pharmaceuticals told TOI.
Favipiravir is an early-use antiviral which showed promising results in certain countries including Japan, but it’s efficacy has been under a cloud in India. Irrespective, the drug is being prescribed and used widely in Covid-19 therapy across the country, with its sales seeing a massive surge during the second wave.
The antiviral drug was approved in June by India’s drug regulator under `restricted emergency authorisation’ under the accelerated approval process, for mild-tomoderate cases.
Over 30 companies are now producing Favipiravir, including Sun Pharma, Cipla, Abbott, Alkem, Dr Reddy’s, Hetero, Brinton, Jenburkt, Lasa Supergenerics, Delcure and Strides Pharma. Glenmark has also tweaked the strength of Fabiflu to reduce the pill burden, and is now offered in 400 and 800 mg options.
Online sales
2018: Banned by Delhi HC, for entire country
Abhinav Garg, HC bans online med sales across country, December 13, 2018: The Times of India
The Delhi high court ordered a ban on sale of online medicines by e-pharmacists across the country and directed the Centre and the AAP government to immediately implement the order.
A bench of Chief Justice Rajendra Menon and Justice V K Rao passed the order while acting on a PIL filed by Delhi-based dermatologist Zaheer Ahmed who complained that lakhs of medicines were being sold on the internet every day without much regulation, posing a huge risk to patients and doctors alike.
In the plea filed through advocate Nakul Mohta, Ahmed pointed out that online sale of medicines is not permitted under the Drugs and Cosmetics Act, 1940 and Pharmacy Act, 1948.
The petitioner highlighted that even though the Drug Controller General of India in 2015 clearly directed all state drug controllers to protect the interest of public health by restraining such sale online, lakhs of medicines continue to be sold online, often even without prescription.
Online sale of drugs can lead to misuse: PIL
Unable to supervise, the government has failed in its responsibility to protect public health which is its constitution obligation under Article 21, the PIL says.
“Unlike common items, drugs are highly potent and its misuse or abuse can have serious consequences on human health, not just for the person consuming it but for humanity at large as some drugs can be addictive, habit forming and harmful to the body. A large number of children/minor or people from uneducated rural background use internet and can be victims of wrong medication while ordering medicines online,” the PIL argues, seeking the court’s intervention.
Blaming the government for not doing enough, the plea says online pharmacies are operating without a drug licence and warns that “unregulated sale of medicines online will increase the risk of spurious, misbranded and substandard drugs being sold” adding that “Some drugs have psychotropic substances and can be easily ordered on internet and misused for criminal activities or drug abuse.”
The PIL states that Centre is well aware of the risks involved in sale of medicines on internet since a panel setup by it for this purpose cautioned as late as September this year about risks involved in the online sale of medicines, particularly, prescription, habit-forming and addictive medicines.
In September the Union health ministry had come out with draft rules on sale of drugs by e-pharmacies with an aim to regulate online sale of medicines across India and provide patients accessibility to genuine drugs from authentic online portals. The draft rules on “sale of drugs by e-pharmacy” state that no person will distribute or sell, stock, exhibit or offer for sale of drugs through e-pharmacy portal unless registered.
The plea said unregulated sale of medicines by online pharmacies would increase the risk of spurious, misbranded and substandard drugs being sold
Opioids
2025/ Govt. bans manufacture, export of 2 drugs ‘fuelling’ W Africa opioid crisis
Naomi Canton & Eshan Kalyanikar, TNN, February 24, 2025: The Times of India

From: Naomi Canton & Eshan Kalyanikar, TNN, February 24, 2025: The Times of India
London/Mumbai: A media exposé on a Maharashtra-based pharma company accused of illegally manufacturing unlicensed, addictive opioids and exporting them to West Africa led to a joint raid by state and central drug inspectors over the weekend and the announcement of curbs on the manufacturing and export of such products.
The raid on Aveo Pharmaceuticals Pvt Ltd’s facility and warehouse in Boisar under Palghar district followed a BBC World Service documentary titled ‘India’s Opioid Kings’. It examined the public health crisis caused by pills made of a deadly cocktail of tapentadol (a powerful opioid) and carisoprodol (an addictive muscle relaxant), packaged as legitimate licensed medicines, in countries such as Nigeria and Ghana.
This drug combination is not licensed for use anywhere in the world. “They are coming in from India and that has become a big menace in Nigeria,” a member of Nigeria’s drug enforcement agency told BBC.
Maharashtra FDA officials said the entire stock was seized, and further production was immediately halted. The company was also slapped with a showcause.
On Friday, the Centre issued directives to all states to revoke the no-objection certificate (NOC) for export and the manufacturing licences for tapentadol, carisoprodol, and other similar products.
Speaking to TOI, an FDA official said, “Aveo has been on our radar for the past few months. We served them notice in October for nonmatching of their manufacturing and distribution records. Central Drugs Standard Control Organization (CDSCO) is responsible for testing the exports as per their protocols, and the state does not play a role. Both the medicines have their use separately, but their combination is harmful, and it is already banned in India.” Monish Bhalla, former senior officer of India’s Nar- cotics Control Bureau, said, “The checks and balances on imports in India are far more stringent than for exports.”
Aveo is breaking Indian law because it is not meeting import requirements in Ghana, but “the Indian authorities pay little attention to pharma drugs not sold in India,” the documentary states.
An undercover BBC reporter went to Aveo Pharmaceuticals’ factory posing as a businessman looking to supply opioids to Nigeria and met the managing director Vinod Kumar Sharma. Whilst being plied with snacks and Bisleri, Sharma showed him the pills boxed under different brand names such as Taamdol 225mg and Taramaking 250, all made from the same dangerous cocktail.
Sharma told the reporter, “This is very harmful for their health, but the customer cannot understand that. He wants to relax. Any medicine can be misused. This is a very harmful product in their hands, but nowadays this is business. I can clear from our customs, means India. You can clear from your side.”
He told the reporter that he can ship them to Ghana and they can enter Nigeria from there. The drugs he shows are the same ones that have been seized by the police in West African nations because they are illegal. Sharma also boasted that his factory is “WHO certified”.
The documentary also focuses on a drug rehabilitation centre in Nigeria where patients addicted to these opioids live in appalling conditions with their legs chained together, sleeping on the floor with no running water. In Tamale, Ghana, local city chief Alhassan Maham has created a citizens’ task force of 100 volunteers whose mission is to raid and arrest the drug dealers and take these illegal opioids off the streets. But the problem is: India is manufacturing them faster than they can do that.
CDSCO told the BBC it has taken the matter up with countries in West Africa and will take immediate action against any pharmaceutical firm involved in malpractice.
While Sharma did not respond to TOI requests, the company in a statement said that the allegations against it were baseless and without merit. The company added that it adhered to the rules and regulations set by various regulatory authorities to manufacture and export its products.
Pharma GCCs
As of 2025
Swati Bharadwaj & Rupali Mukherjee, TNN, Sep 19, 2025: The Times of India
India is already called the ‘pharmacy of the world’ for manufacturing quality and affordable medicines. To be counted as a drugs powerhouse, it has to now find a way to move up the value chain. Whatever the approach, if pharma GCCs, or global capability centres, located in the country appear vital to the journey, it’s because they’ve come a long way themselves.
Over 55 healthcare & lifesciences (H&LS) GCCs are now present in India, operating over 95 centres spread across mainly Bengaluru, Hyderabad and Mumbai. Together, they represent millions of dollars in investment and lakhs of roles, including ones increasingly in research and drug development. Among those who’ve trooped in are as many as 23 of the world’s top 50 life sciences companies. While about half of these made their entry only over the last five years (according to a recent EY India report), it’s a story that begins almost a quarter century back.
Growth Momentum
2001. Just after the turn of the millennium, when India was basking in the success of its Y2K moment that heralded its arrival on the global IT stage, pharma giant Novartis planted the seed of what would become the next big offshoring story for India. The Swiss company put together a modest 20-member team in Mumbai to test the waters for building capabilities in data management, statistics and IT.
Cut to 2025, and Novartis Corporate Centre (NCC) India is the largest pharma GCC in the country. In between came a move to Hyderabad in 2007-08 and investments of more than $400 million. NCC India now houses over 8,500 employees, including around 2,800 people in development across Hyderabad and Mumbai. Ganpat Anchaliya, head of NCC India, says “foresight and conviction” led them to act “well ahead of peers”. But it didn’t take very long for others to follow.
As EY points out, what started as a trickle in the 2000s with pharma giants Novartis, Johnson & Johnson, and Novo Nordisk gathered pace in the mid-2010s as Eli Lilly, Merck KGaA and Bayer headed to Indian shores. Post Covid-19, this momentum has only accelerated, with pharma MNCs gearing up for a coming decade of uncertainty, high competition and increasing regulatory scrutiny.
Why India?
India, experts say, has become crucial for big pharma as the industry stares at a $236 billion patent cliff between 2025 and 2030, when nearly 70 blockbuster drugs go off patent.
“India combines three strengths that are rare to find in one location — sheer scale that gives it global relevance, talent, and impact,” says John Dawber, corporate VP & MD of global business services at Danish giant Novo Nordisk. If India’s large pool of scientific, medical, and digital talent — more than 2.7 million professionals in life sciences and a strong pipeline of over 2 million STEM graduates annually — is virtually unmatched, it has also succeeded in leveraging other strengths.
Anil Matai, director general of the Organisation of Pharmaceutical Producers of India (OPPI), says what makes India unique is its positioning at the convergence of world-class scientific talent, robust digital infrastructure and a thriving innovation mindset. “Earlier it was a generic copycat mindset but, now, the mindset is also changing and we see the scales tilting towards innovation. Also, for global clinical trials, we have a large patient pool as well,” says Matai, who was at the helm of Novartis India when its GCC moved to Hyderabad.
Sairam Nair, lead, GCC, and country president, Sandoz India, sees the pharma GCC boom as the result of a unique blend of cost efficiency, deep domain expertise, mature operating environment, and a tech- and AI-native talent pool. But not all are convinced.
Doubts And Concerns
Some feel that though GCCs have achieved much, especially in scaling talent, they are yet to do any fundamental innovation in India.
“They (GCCs) are largely back offices for global innovators, who moved the routine work here first and now are adding more layers to it. But I still don’t think they’re doing fundamental innovation here. I look forward to the day when the core of innovation shifts here and they become innovation centres in drug discovery,” says G V Prasad, cochairman & managing director, Dr Reddy’s Labs.
Shiv Kumar, HR head of Merck India, agrees that “from a drug discovery perspective, I think we have some way to go”, but his diagnosis of the problem is different. “Let’s be honest, I think IP (intellectual property) laws in India, while they are evolving, haven’t evolved to a point where there is a lot of confidence,” he feels.
Matai of OPPI does not see it that way — he says a few GCCs have even begun investing in basic research in India — but acknowledges IP issues. “While IP protection has improved, some concerns remain about enforcement and regulatory data protection, which can affect willingness to invest in basic research.” India, Matai says, needs to build on innovation atop its strong foundation of generics, but much will depend on how much comfort Indian regulators are able to provide to MNCs. “If they provide comfort now, then, in the next five to 10 years, you will see significant investment.”
However, Arindam Sen, partner and GCC sector lead, EY India, insists that the shift from cost arbitrage to capabilityarbitrage is real and irreversible. “This isn’t about cost arbitrage anymore, it’s about India becoming indispensable to the global R&D pipeline. Life sciences MNCs are embedding their most strategic, knowledge-intensive work here, making India the epicentre for life sciences innovation, compliance, and future growth.” bb Tech Forward
The shift in approach is already visible in scale and scope with the next leap almost certain to be powered by artificial intelligence (AI).
“Early pilots in Indian GCCs are already showing what’s possible: AI-based molecule triaging that saves up to nine months in discovery cycles, predictive safety systems in pharmacovigilance, and machine-learning models that optimise clinical trial design,” says pharma expert Sujesh Vasudevan, a senior adviser at BCG.
“Novartis in Hyderabad is applying AI to anticipate drug safety outcomes, while AstraZeneca is deploying machine learning to identify novel cancer drug targets. Reports suggest that such models can reduce discovery costs by as much as 70% while cutting timelines by nearly in half. For global pharma, India’s GCCs now offer not just scale but speed, insight, and innovation,” he adds.
The AI pivot is already at work. “Our hub in Hyderabad integrates cutting-edge tech like AI, machine learning, and software engineering to accelerate drug discovery, optimise clinical trial processes, and generate actionable market insights,” says Pranab Kakati, head of data solution implementation services, data science & AI, Bayer Pharmaceuticals Private Ltd. Nair of Sandoz GCC says they’re “exploring smarter, practical ways to integrate AI into operations to deliver impact that’s both meaningful and sustainable”.
According to Bhanu Prakash Kalmath SJ, partner and healthcare industry leader, Grant Thornton Bharat, the infusion of AI, particularly in knowledge- and data-intensive processes, is expected to further deepen the transformation and enhance Indian GCCs’ role in global innovation.
Are We There, Yet?
Attesting to this, Anchaliya of Novartis says their over 8,500 associates anchored in Hyderabad are not only advancing scientific, digital insights, and tech frontiers but also shaping how the company operates globally.
Matai of OPPI, which represents research-based global pharmaceutical MNCs in India, says “the shift has been dramatic in the last few years”.
“While it was more about cost optimisation in the early days, the needle has moved significantly and, now, a lot of highend work is getting done here. Today, we have 16 member companies that have set up GCCs in India,” he says. Matai says that while “(Novartis) were the early ones to spot the opportunity”, EY India’s Sen sought to underline the sector’s rapid evolution. “In just five years, GCC penetration in enabling functions like finance, HR, supply chain, and IT has crossed the 60% mark, but what truly stands out is their deepening role in core functions — from drug discovery and regulatory affairs to medical and commercial operations,” he says.
THE GCC TRIANGLE: MUMBAI, BENGALURU, HYDERABAD
India’s first pharma GCC, of Novartis, opened in Mumbai in 2001. But Bengaluru leads the pack now with 33% of these GCCs based there. The city making waves now is Hyderabad. Post Covid, practically every GCC that has opened in India has come up in the Telangana capital.
■ The more than 55 pharma GCCs that employ over 3 lakh professionals mainly operate out of these three cities, which together account for nearly 75% of the total installed sectoral talent, according to GCC enabler ANSR.
Novartis’s move to Hyderabad’s new IT district has worked as a magnet, bringing the likes of BMS, Sanofi, Eli Lilly, Bayer, Evernorth, Medtronic, Providence, among others, to the city, which houses GCCs of eight of the top 10 global pharma companies.
THE GCC BRIGADE
Novartis
India’s first GCC started with a 20-member team in Mumbai in 2001 and also set up in Hyderabad in 2007-08 with an investment of more than $400 million coming over the past two decades. India’s largest pharma GCC, it has over 8,500 employees.
Sanofi
Set up in 2019, its GCC has grown to over 3,000 people in Hyderabad. Investments worth €400-million are lined up till 2030 for its GCC, which is driving high-value functions like drug discovery and digital health innovation. Mrinal Duggal, head, Sanofi Global Hub in Hyderabad, said “the Hyderabad hub has become integral to Sanofi’s mission”.
Bristol Myers Squibb
It invested $100 million to open its innovation hub in Hyderabad in 2024 with over 1,500 employees. It’s focussing on applying AI to drug discovery, speeding up clinical trials and regulatory documentation through automation.
Novo Nordisk
Its Bengaluru hub, conceived in 2007, has grown to around 4,500 professionals spanning 17 functions that support everything from early R&D to supply-chain planning. John Dawber, corporate VP & MD of its global business services, said the India hub is leveraging public-private partnerships and advances in AI and automation to enable Novo Nordisk “to accelerate innovation and deliver better outcomes globally”.
Eli Lilly
Started with around 65 employees in 2016 in Bengaluru and has grown to 3,500 people. It recently expanded to Hyderabad, where 1,500 roles are planned. The Bengaluru site plays a key role, among other things, in streamlining clinical trials and drug development. The Hyderabad site will leverage AI, automation, cloud computing, etc.
Merck KGaA
The German giant started its India GCC journey with the acquisition of Sigma Aldrich in 2015. It has grown to three GCCs housing around 2,500 people in Bengaluru. Suneela Thatte, VP & head, healthcare R&D for India, said the “vision is to keep going up the value chain... to see how we get more technical and strategic roles to India”.
Pfizer
Its global drug development centre in Chennai, embedded in IIT Madras Research Park, employs over 250 scientists to deliver advanced APIs, formulations, and devices to global markets.
AstraZeneca
Has steadily expanded from Chennai to a 166-crore global hub in Bengaluru, focused on AI-driven drug discovery and digital health.
Sandoz
The generics giants, which was spun off from Novartis in late 2023, started with just 800 employees and has grown to over 1,000 people in Hyderabad.
Bayer Pharma AG
Came in over a decade ago, but has remained compact by extensively leveraging their partner ecosystem. Its Hyderabad facility is a critical hub for pharma R&D and clinical trial operations focused on advancing oncology and cardiology therapeutics.
Prices of Drugs and pharmaceuticals
Nodal agency: National Pharmaceutical Pricing Authority
The Hindu Business Line, November 26, 2016
S. Srinivasan
NPPA (National Pharmaceutical Pricing Authority), formed in 1997 implements the DPCO (Drug Price Control Order) 2013.
In considering dismantling of the institution, the thought is that price control should be “delinked” from the 370-plus essential drugs. And thereafter price control must be confined to what the government thinks fit. Tell this to the poor people standing in the queue to exchange their demonetised notes. They understand the devastating impact of such a move on their lives.
The argument being given for dismantling price control is that:
a) it inhibits growth of pharma industry and
b) inhibits new investment.
This is a wrong argument and not evidence based. Price control affects only 12 per cent (maximum of Rs. 12,000 crore) of the total domestic market of more than Rs. 1 lakh crore. Out of which less than 50 per cent of the “affected” market, that is only Rs. 6,000 crores, had to actually reduce their prices to the ceiling price fixed by the DPCO. About 88 per cent of the market is out of price control. During the period DPCO 2013 has been in operation, the domestic sales of medicines have increased from Rs. 70,000 crore in 2013 to more than Rs. 100,000 crore as of date. Exports are another Rs. 100,000 crore. So, does the DPCO really inhibit growth?
There is in fact a need to expand the span of price control to cover a range of essential and life-saving drugs as directed by the Supreme Court in its order dated March 10, 2003 in Union of India vs KS Gopinath and Ors. The Supreme Court has clearly held that the government has a Constitutional obligation to ensure the affordability of essential medicines. The AIDAN (All-India Drug Action Network) and Others had impleaded themselves in the matter in 2003 and the case is still before the Supreme Court (Writ Petition (Civil) 423/2003). The current DPCO 2013 is itself an outcome of the above PIL. It is, hence, curious that the Government should discuss the dismantling of the DPCO 2013 when:
a) the matter is sub judice, and
b) when it is against the spirit of Supreme Court directives on the issue.
AIDAN has served a legal notice, citing the case, to those involved with the discussion of dismantling the NPPA (the CEO of the Niti Aayog and the Secretaries of Health, Department of Pharmaceuticals and the Department of Industrial Policy and Promotion).
Pharma industry lobbies are reeling from two recent court orders: one, the refusal by the Bombay High Court to question the legitimacy of the NPPA’s action, under Para 19 of the DPCO 2013, that brought more essential drugs, like antidiabetics and cardiovasculars, under price control. The other order, by the Supreme Court, will result in industry having to pay more than Rs. 4,500 crore for overcharging essential medicines under the previous DPCO. It is no wonder that the very foundation of price control is now being brought to question.
But in the interest of bringing in greater “ease of doing business”, the Government needs to ensure that Parliament and the regulatory requirements are not bypassed, stakeholders are not kept in the dark, and patient interest is not sacrificed.
Trends
Limited rural reach inspite price control measures
The Times of India, Jul 15 2015
Sushmi Dey
Pharma price control has stunted innovation: Study
Lower cost but rural reach poorer
Consumers may be happy about a cut in medicine bills but the government's price control measures have forced many brands out of the “unviable“ pharmaceutical market, resulting in a drastic slowdown in launches over the last five years. From an average of four new drugs being launched in any specific category in 2011, it was down to a mere one in 2014-15, implying a 75% decline in launches, according to estimates by IMS Health -a leading healthcare market research agency .
Not just that, data collected since 2013, when the new pharmaceutical pricing policy came into place, show a sharp decline in consumption of price-controlled medicines. That's because of a growing push for alternative options outside price control.
With lower margins in price-controlled medicines, there is also less incentive to reach out to rural markets. “For low-income households that are reliant on the government system for healthcare, DPCO (Drugs Price Control Order) will not improve the patient's ability to purchase drugs. This is supported by the fact that no significant penetration of price-controlled molecules in rural markets is visi ble...,“ says a new report, Assessing the Impact of ` Price Control Measures on Access to Medicines in India' by IMS Health.
According to the report, consumption of price-controlled medicines in rural areas dropped by 7% in the past two years, whereas sales of other medicines increased by 5%. It says even in Tier-II and III cities, such medicines have witnessed a muted growth.
The industry argues that the move has failed to achieve the intended objectives of increased affordability and availability because policy measures have impacted tail-end brands more than the leading players.But public health experts brush aside such concerns saying companies manufacture one medicine under several brands with different compositions, and regulations are aimed at bringing them on a par in terms of pricing.
Life-saving medicines: limited competition keeps rates high
Rupali Mukherjee, Critical illness drugs remain unaffordable, Nov 03 2016 : The Times of India
Steps To Cap Prices Of Life-Saving Meds Prove Futile, Limited Competition Keeps Rates High
Market dynamics and limited competition in biosimilars (approved versions of complex biologic drugs) and other life-saving drugs have resulted in little reduction in prices, making them unaffordable for many . Surprisingly , government measures to bring down their prices (through the Drug Price Control Orders, or DPCOs) too have been futile, making them out of reach for patients as treatments often run into lakhs of rupees. Biosimilars are used in cancer therapy , rheumatoid arthritis and other critical illnesses.
Even in the case of medicines for hepatitis B and C like sofosbuvir, tenofovir and entecavir, which were brought under price control, there has been little change in the access scenario. Take the example of two biosimilars -rituximab and trastuzumab -where there is not much change in MRP , even after government brought these drugs under price control in April and May respectively .The National Pharmaceutical Pricing Authority (NPPA) ceiling price for breast cancer drug trastuzumab is Rs 55,812 (440 mg), marginally lower than that charged by companies at around Rs 58,000, while the ceiling price of another cancer drug rituximab (500 mg) is Rs 36,947, close to the MRP of Ristova (innovator Roche's brand) at Rs 37,500.
Ceiling prices are fixed by NPPA based on a simple average of price to retailer (MRP) of products of companies with over 1% market share. “Given this methodology , wherever the innovator has had an almost monopoly position, the price reduction before and after price control has not been significant. Therefore, given the same methodology being in force, a ceiling price revision after two years can be expected to bring about a further reduction consequent to more players participating in the market and offering products at competitive prices,“ K V Subramaniam, president and CEO, Reliance Life Sciences, said.
Leena Menghaney , treatment activist and lawyer, said, “The system devised by NPPA of fixing prices encourages companies to artificially keep prices high, and hence, works against patients and consumer interests. The government has allowed both multinational and generic countries to profiteer out of the disease of people, driving millions of patients to death.“
The cost of production should be taken into consideration, where medicines included in the National List of Essential Medicines (NLEM) are linked to price control. This is because there is evidence that cost of producing certain offpatent registered drugs is cheap, based on cost of active pharmaceutical ingredient (raw material). “The market is not 100% perfect and, at times, anomalies creep in. In certain cases, there are limitations in making drugs affordable,“ an NPPA official said. In the case of hepatitis medicines, pharmacologist Andrew Hill from Liverpool University has calculated the treatment costs for a patient.“The drug entecavir is very cheap, and could be made for only $36 per year, or 10 cents per day . Sofosbuvir can be made for $1 per day , including a profit margin. So the ceiling price fixed by the government seems high,“ Dr Hill told TOI.
“The cost of biosimilars needs to be viewed in the con text of global prices of these expensive biologics which are being provided in India at a fraction of their original cost. The cost of developing a biosimilar ranges between $50-150 million in comparison to a generic which costs around $3-5 million. The scale-up of manufacturing is extremely difficult and expensive,“ Kiran Mazumdar Shaw, CMD, Biocon, said.
However, there is a section which feels that price control alone cannot improve access. “Competitive pricing, local production and compulsory licensing will drive down prices“, says Indian Pharmaceutical Alliance secretary general D G Shah.
’Retail margin on generic drugs as high as 1,000%'
If you think doctors prefer to prescribe expensive drugs when cheaper options are available, you are only partly right. A study in Indian Journal of Pharmacology found that while generic versions were less expensive than the branded ones, the retail margin on generic variants was way higher -sometimes more than 1,000% of the manufacturer's price.
This implies that retailers have a strong financial incentive to push generic drugs, even if doctors prescribe branded. PM Narendra Modi had on Monday said that government is contemplating a legislation that will make it mandatory for the doctors to prescribe generic drugs. Authored by pharmacologists from Delhi University's VP Chest Institute and MD University, the 2010 study compares retailers' profit margin on five common drugs -cetirizine, fluoxetine, ciprofloxacin, lansoprazole and alprazolam.
Dr Anita Kotwani, professor of pharmacology at Delhi University's V P Chest Institute, told TOI fleecing of patients in this manner continues. “It will not stop until government comes up with regulation to ensure manufacturers drop trade names and sell drugs by their pharmacological name as in the US and other developed countries,“ she said.
Dr Kotwani was the lead author of the IJP study . She said retailer margin for five branded medicines they studied was in the range of 25%-30%, but for their branded-generics version manufactured by the same company it was in the range of 201% -1,016%. “In India, there are very few innovator or patented products. Generic drugsare available in two forms: the branded product which companies advertise and push through doctors and branded-generic which they expect retailers to push in the market,“ she said.
In the IJP study , quality of both branded and branded-generic drug was found to be equally good. But doctors say all generic drugs sold under different name do not have similar quality. “We have observed that epilepsy goes out of control in epilepsy patients when they changed the brand which is indicative of variation in quality of drugs manufactured by different companies,“ said Dr Kameshwar Prasad, profes sor and head of neurology at AIIMS. He added there should be strict control on quality and pricing of generic drugs.
“Doctors are often blamed for coming under the influence of big firms, who lure them with different benefits, and batting for brands. This is partially true. The fact that quality of generic drugs sold under various trade names for same molecule is not uniform, hence lack of trust on the less popular ones,“ said another doctor.
Ironically , India is one of the world's largest exporters of generic drugs. It exports to over 200 countries, including the highly regulated markets of US, Europe, Japan and Australia. But the experts say quality control is much better abroad. “There are only 1800 drug inspectors in the country .This number is grossly inadequate,“ Dr K K Aggarwal, president of Indian Medical Association, said. He added that less than 0.01% of the drugs produced in the country are tested for quality. “For doctors to prescribe generic drugs, it is crucial that the laws regarding drug testing and quality assurance are strengthened,“ the IMA president clarified.
Prime Minister Modi, while speaking at a function to inaugurate a charitable hospital in Gujarat on Monday , said the government will bring in a legal framework by which if a doctor writes a prescription, he has to write in it that it will be enough for patients to buy generic medicines and he need not buy any other medicines.
WHO, 2019: Many essential drugs priced very high
From: Sushmi Dey, ‘Many essential drugs priced much higher than mfg cost’, April 17, 2019: The Times of India
WHO Study Reveals Profiteering By Cos, Scope Of Cutting Prices
Around 40% of the essential medicines in India with lowest MRP are priced significantly higher than estimated production costs, an assessment by the World Health Organisation (WHO) shows highlighting the “exorbitant” profiteering by pharmaceutical companies and the scope for lowering prices of drugs.
While innovative and newer drugs for cancer, hepatitis C and rare diseases are out of reach of many due to their unaffordable prices, even off patent drugs which are in market for long and commonly used for diseases like HIV, tuberculosis and malaria are priced very high with huge margins over their cost of production.
This results in high expenditure pushing people into poverty. In India over 75% of health expenditure is out-of-pocket, of which the major chunk is spent on medicines. This is despite India being a manufacturing hub and the biggest supplier of low cost generic medicines to the world.
The study shows while Indian prices were below WHO’s estimated generic price in many cases, they were mostly government tender prices, which are likely to be significantly lower than the private market prices more often experienced by those needing medicines in India.
Moreover, most of the high-priced medicines in India were found only in the private market, suggesting a lack of availability in public facilities, the study said.
Findings of the report were discussed by the global health community at an international forum hosted by the UN agency on fair pricing and access to medicines last week in Johannesburg.
Delegates from governments and civil society organisations called for greater transparency around the cost of research and development as well as production of medicines, to allow buyers to negotiate more affordable prices. “The market prices of medicines in India do not bear any resemblance to the cost of production. Unfortunately, the national pricing regulation, applied to essential medicines, has shifted from a cost-plus mechanism to a market-based mechanism in 2013. This has been challenged by AIDAN in the Supreme Court because the market-based system is deeply flawed,” says Malini Aisola of All India Drugs Action Network (AIDAN).
India vis-à-vis the world
2019: Indonesia, Thailand were cheaper

From: Nov 23, 2019: The Times of India
See graphic:
2019: Drugs and Pharmaceuticals prices in India vis-à-vis Indonesia, Thailand and the world
USA and Indian pharmaceuticals
2017: 300 USFDA generic approvals, a record
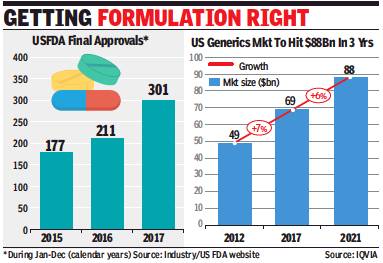
ii) The size of the US market for generics, 2012-17
From: Rupali Mukherjee , Indian pharma cos get record 300 USFDA generic drug nods, January 25, 2018: The Times of India
See graphic:
i) USFDA Final Approvals, 2015-17; and
ii) The size of the US market for generics, 2012-17
Domestic pharma companies received more than 300 approvals in 2017 to launch generic drugs in the US, which is an all-time high. The clearances came despite regulatory pressure from the US Food and Drug Administration (FDA), and unprecedented warning letters issued to the pharma companies’ facilities.
The final approvals for Indian players are up by nearly 43% from 211 in 2016, and corner about 40% of all global filings in the highly lucrative around $70-billion US market. This, even as all drug biggies — including Zydus, Sun Pharma, Dr Reddy’s and Cipla — faced regulatory ire, while some were pulled up for manufacturing lapses by the US regulator during last year.
In terms of each company, Zydus leads with 66 approvals in 2017, followed by Aurobindo (52), Glenmark (18), Lupin (17), Gland Pharma (16) and Cipla (10). Zydus cornered a majority of US filings as its Moraiya facility, which contributes about 60% of US sales, came out from under USFDA scanner in June last year. Sun Pharma remained static at 10 approvals, due to its Halol plant continuing under the regulatory glare.
The US generics market, a key driver of Indian pharma’s growth, has always been a dynamic market. But the pace of change has accelerated in the last few years. The increase in competition and consolidation of distribution channels have led to the US generics business getting commoditised. Price erosion has been at an all-time high and this has impacted operating margins significantly. Major domestic companies earn at least 40% of their overall sales from the US.
To maximise margins, companies are now launching complex generics and speciality products.
Glenmark Pharma chairman & MD Glenn Saldanha says, “While the number of ANDA (abbreviated new drug application) approvals continue to remain strong for us, we, however, have begun the process of transitioning the US business to a specialty/innovation business. While the generics business in the US will still grow, the future for us will be in the specialty and innovation business. We plan to file our first specialty NDA in the next few months in the US.”
Warnings: 2017-19
Rupali Mukherjee, January 20, 2020: The Times of India

From: Rupali Mukherjee, January 20, 2020: The Times of India
MUMBAI: The Indian pharma industry, which is rapped for poor manufacturing practices globally, seems to be cleaning up its act, at least in relative terms. American drug firms were issued 54 warning letters by the USFDA, while India — which had the distinction of getting most warning letters — stood second with 17 such notices in the year ended September (US financial year). China ranked third with 14 warning letters (see graphic).
A company slapped with a warning letter is barred from introducing new products in the US, hindering its ability to increase sales in the world’s top pharmaceutical market. Earlier, emerging economies like either India or China topped the list. In the previous year, the US received 19 warning letters, while China got most at 21, and India had 15.
Data integrity issues, which include inappropriate manufacturing practices and overlooking results while testing medicines, continue to dog the drug industry with 32 of 98 warning letters in FY19 citing the issue. In the previous year, 34 of 94 warning letters were due to data integrity lapses, which have a significant impact on drug product quality.
Several domestic pharma companies have been hauled up by the US regulator over issues like lax documentation, overlooking test results and unhygienic manufacturing facilities with open toilet drains. Indian companies command around 35% volume share in the highly lucrative US generic market, valued at nearly $70 billion.
“Common issues for manufacturing establishments around the world (including the US, India and China) include inadequate or poor quality systems implementation, data integrity issues, inadequate validation of various processes used in manufacturing or testing, and product contamination. In any region, some drug manufacturers meet US requirements, while others do not. When we determine that there are significant violations at a manufacturing establishment, we take appropriate action to protect the public health,” a USFDA official told TOI.
The official added, “It is important to note that the number of annual inspections in any region or country may fluctuate year-to-year because some inspections occur on a routine basis, while others take place when the FDA is reviewing specific product marketing applications, or when the agency receives information about potential product manufacturing or quality problems.”
In 2019, major companies — including Lupin, Torrent, Aurobindo, Strides Pharma, Cadila Healthcare, Indoco Remedies, Glenmark, Jubilant Life Sciences and Emcure Pharma — were issued warning letters for significant violations of current good manufacturing practice (CGMP) regulations. Typically, the action follows inspections and audits carried out by the US regulator.
PwC India leader (pharmaceutical & life sciences) Sujay Shetty said, “Data shows the phenomena of warning letters spread across many countries, and not just unique to India. Indian companies hog the limelight, though they may have got lower number of letters (in other years). Also, the data speaks volumes about domestic companies investing effort and resources in upgrading quality systems in their facilities.”
Commenting on USFDA audits, Sun Pharma managing director Dilip Shanghvi had said in a recent investor call, “Indian companies file large number of products, and as a part of their assurance to the US Congress, the USFDA has said that they will try and conduct as many pre-approval inspections as necessary. If we continue to be the largest filer of new ANDAs in the world, we will have a large number of inspections. As regulators see any kind of a risk in one inspection, they add as part of the inspection plan for future audits. Accordingly, the focus of USFDA inspections will continuously change, and it will help improve the quality of products for safety of patients.”
2019: 7 Indian companies face lawsuits from 44 US states
Rupali Mukherjee, May 14, 2019: The Times of India
Over 40 US states filed lawsuits against pharma biggies, including seven domestic companies, over collusion in inflating prices of widely-prescribed generic medicines, in certain cases as high as 1000%.
The 500-page case filed by US states on May 10, charged global biggies like Teva, Pfizer, Sandoz and Mylan, as well as domestic companies like Sun Pharma’s US arm Taro, Zydus, Lupin, Aurobindo, Dr Reddy’s, Wockhardt and Glenmark of conspiring to inflate prices of medicines, and stifling competition for generic drugs. The lawsuit widens a complaint of price collusion filed in 2016, which is still pending in US courts after being first investigated in 2014. Domestic companies said they would defend these allegations. A Sun Pharma spokesperson said “We believe the allegations made in these lawsuits are without merit, and we will continue to vigorously defend against them.” The US probe on price fixing seems to have impacted overall domestic pharma sector, with stock prices of most companies witnessing a drop on the BSE of 4-9% on Monday. Sun Pharma scrip tanked the most by nearly 10% to Rs 397.
The domestic industry, already grappling with pricing pressure in US, faces one of the biggest such probes ever, which if proven can incur litigation and financial burden on companies, experts say. The development put a dampener on the entire sector, as US is the most lucrative market contributing a significant portion to revenues. “The role of domestic companies (under investigation) appears to be minimal, probably to the extent of three to four molecules, while we expect the penalty would not be over $50 million each”, Surajit Pal associate vice-president, Prabhudas Lilladher told TOI. Over years, the industrywide scheme reportedly affected prices of over 300 generic drugs in the US including HIV, diabetes, asthma medication, high cholesterol, oral antibiotics, blood thinners, cancer drugs, contraceptives and antidepressants. The 20 drug companies reportedly engaged in illegal conspiracies to divide up the market for drugs to avoid competing and, in some cases, conspired to either prevent prices from dropping or to raise them, according to the complaint by 44 US states, filed in US district court in Connecticut.
Soaring drug prices from both branded and generic manufacturers have sparked outrage in US, with criticism coming from across the political spectrum, including from President Donald Trump.
Vaccine production
India’s place in the world
2020
From: January 11, 2021: The Times of India
See graphic:
Vaccine production capacity in India and the world, 2020
See also
Drugs and Pharmaceuticals: India