Drugs, pharmaceuticals, medical devices made by Indians
This is a collection of articles archived for the excellence of their content. |
Contents |
Adhesive for bone and skin repair
Oct 28, 2023: The Times of India
BHOPAL: Think of a magic adhesive that sticks things underwater, binds bones and skin and even repairs your broken chair. Scientists at the Indian Institute of Science Education and Research, Bhopal, and School of Medical and Allied Sciences, Haryana, have developed just such a stick-anything glue. What's more, it's biodegradable.
The adhesive, named 'A30' as of now, has the potential to "heal and repair injured and dissected tissues", doing away with the need for painful sutures and staples.
It can join broken bones and can even be used to make drug delivery more effective, says the study published in the peer-reviewed 'Chemistry - A European Journal'.The research was conducted by Professor Aasheesh Srivastava and Dr Tanmay Dutta of IISER, and Dr Aashish Sharma of School of Medical and Allied Sciences. They have obtained an Indian patent for the adhesive.
The 'clear synthetic biomedical adhesive' is not only biodegradable but also biocompatible, meaning it's not harmful or toxic to human tissue. It can bind various surfaces, such as tissues, bones, eggshells, and wood in both air and underwater, said Srivastava, adding it hardens by itself without requiring additional chemicals.
Cancer detection
2019/ Test to detect spread or relapse
Umesh Isalkar, August 21, 2019: The Times of India
A simple blood test costing Rs 15,000 will soon help doctors detect the spread or relapse of cancer in patients, helping them to plan an efficient treatment module. The test, based on a liquid biopsy technology called OncoDiscover, will help pick up circulating cancer cells in a patient’s blood much before they have affected or spread to other body parts.
Developed by Pune-based scientist Jayant Khandare, the diagnostic tool may also be used to plan treatment or to assess how well the existing treatment regime is working. “The test will be launched in a phased manner all over India from September,” Khandare told TOI.
‘Indian cancer test kit will be far cheaper than the US version’
The long-term plan is to release the test in key global markets within the next few years,” Khandare told TOI.
Khandare is the chief scientific officer at Actorius Innovations and Research Pvt Ltd, a startup co-founded by him and Aravindan Vasudevan in 2013. The startup has been funded for high-risk innovation by the Biotechnology Industry Research Assistance Council, an industry support wing of the department of biotechnology, Union ministry of science and technology.
Khandare said the technology was among the few laboratory-to-clinic translational researches, a rarity in India. “OncoDiscover can allow regular monitoring of disease progression. It can also help in providing an early indication for cancer relapse. It is a blood test that detects circulating tumour cells even when their presence is extremely low or minuscule in a patient’s blood,” he said.
The Central Drugs Standard Control Organisation, the regulatory body for drugs and medical devices, has already granted a licence for the manufacture and sale of the test kit in India under the Medical Device Rules, 2017.
Khandare says it is the second such technology in the world, after the one already available in the US. The Indian version would be far cheaper and more specific than the US one, he said. “CellSearch, the US test for the detection of circulating tumour cells has been sold to Menarini Biotech by Johnson & Johnson. It has been available in the US since 2004 and has the approval of the US Food and Drug Administration. The test costs between Rs 84,000 and Rs 1 lakh and is not available in India,” Khandare said.
Cancer treatment
Cytotron/ 2019
Chethan Kumar, Nov 12, 2019: The Times of India
The US Food and Drug Administration (FDA)’s Centre for Devices and Radiological Health has designated a medical invention by a Bengaluru-based scientist as a “breakthrough device” in liver, pancreatic and breast cancer treatment.
Cytotron, developed by Rajah Vijay Kumar, aids in tissue engineering of cancer cells, altering how specific proteins are regulated to stop these cells from multiplying and spreading.
“We are pleased to inform you that your device and proposed indication for use meet the criteria and have been granted designation as a breakthrough device,” states a communique from the FDA wing to Shreis Scalene Sciences, the company that had taken the device to the US.
‘Anti-cancer device to be manufactured in India’
Cytotron is intended to cause degeneration of uncontrolled growth of tissues. “It is indicated for treating protein-linked, abnormally regenerating disorders such as neoplastic disease, and allowing extended progression free survival, with pain relief, palliation, improved quality and dignity of life,” says the letter.
Rajah Vijay Kumar had developed Cytotron at the Centre for Advanced Research and Development, which is headquartered in Bhopal, after nearly 30 years of research into cellular pathways and interactions with specifically modulated fast radio bursts.
“It is a great feeling that after so many years of hard work, against all odds, an institution like the USFDA is designating our work as a breakthrough in the treatment of three types of cancers,” Kumar said.
New technologies in the battle against cancer have generally been hard to come by. It’s even rarer for an Indian device to get breakthrough status in the US. The Centre for Devices and Radiological Health is responsible for pre-market approval of all medical devices in the US, ensuring they are safe for use and effective.
“The devices will all be made in India, given that there are hardly any imported components. And our American partner will take the device to the US. Cytotron is already an approved medical device and is in use in the UAE, Mexico, Malaysia and Hong Kong, among others,” Kumar said.
Turmeric- based curcumin
Rajiv G, January 14, 2020: The Times of India
THIRUVANANTHAPURAM: A potentially breakthrough cancer-fighting technology involving a molecule extracted from turmeric has won Thiruvananthapuram’s Sree Chitra Tirunal Institute for Medical Sciences a US patent.
According to Lissy Krishnan, head of Sree Chitra’s research team, delivery of “curcumin” directly to the affected tissues rather than through conventional oral or intravenous methods enables it to target malignant cancer cells while sparing the healthy ones around them.
Turmeric has proven anti-cancer properties and curcumin, a molecule extracted from it, is easily absorbed by the body and aids blood clotting, Krishnan said. At Sree Chitra, research funded by the Indian Council for Medical Research focused on processing curcumin to form a easy-to-use wafer configuration. When applied to body tissues, the curcumin present in the wafer is released into tissue fluids.
Human albumin, or rich proteins present in the fibrin clot produced from heavy bleeding, binds albumin receptors to cancer cells, thereby permitting its entry into the cells. Simultaneously, the fibrin clot is removed by the body’s natural clot breakdown mechanism without any adverse effect.
“The fibrin wafer is targeted for implantation into the affected site after surgical removal of cancer tissue for killing any remaining malignant cells that have the potential to cause recurrence of cancer or spread to other parts of the body. In addition to drug delivery, the fibrin wafer can promote blood clotting at the surgical site,” said Asha Kishore, director of Sree Chitra.
The institute is ready to transfer the technology for future development of curcumin as an anti-cancer treatment through animal and clinical trials. “The US patent adds value to our efforts to transfer technology and boosts the industry’s confidence in exploring validation and trials with international markets in mind,’’ Kishore said.
Diabetic foot ulcers
Esmolol hydrochloride gel/ 2023
Parul, June 15, 2023: The Indian Express
Can a new foot gel, that’s awaiting a rollout, work on diabetic foot ulcers? A study by the Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, has found that an esmolol hydrochloride gel was far more effective than standard therapy protocols in use till now.
Sixty per cent of patients had wound closure within 12 weeks with esmolol compared to only 41 per cent with existing care routines, suggesting 2.1 times increase in chances of wound closure with esmolol. Overall, more than three-fourths of patients had complete wound healing with esmolol compared to only half with existing treatments. More importantly, esmolol led to wound healing in a certain group of patients with low haemoglobin, renal problems and low albumin, all of which slow down recovery.
“Esmolol is a short-acting beta-adrenergic receptor blocker approved by the US Food and Drug Administration. Beta blockers are traditionally used in cardiology and specifically esmolol, which has a very short duration of action, is used as an intravenous drug in tachycardia (diseases where heart rate is fast) in cardiac ICUs. But we found some prior literature that esmolol has a beneficial effect in reducing oxidative stress that may improve wound healing. We first repurposed esmolol for topical application and studied how it can be made in various gel concentrations. The findings indicate that topical esmolol may be an appropriate addition to the care protocol for treating diabetic foot ulcers. This can change the way we treat diabetic foot wounds. Esmolol gel can become the standard of care for physicians taking care of diabetic patients with foot ulcers. The drug has been patented by Dr Sudhir Kulkarni, the co-investigator, and will soon be marketed after regulatory approvals. It shall be included very soon in the guidelines for the management of foot ulcers,” says Dr Ashu Rastogi, Associate Professor, Department of Endocrinology and lead author of the study, “Topical Esmolol Hydrochloride as a Novel Treatment Modality for Diabetic Foot Ulcers: A Phase 3 Randomised Clinical Trial.”
The results of the study were presented by Dr Rastogi at the European Diabetes Congress at Stockholm, Sweden, in September 2022 and were very much applauded. The final results of the study have been published recently in the prestigious peer-reviewed journal JAMA Network Open.
Diabetic Foot Ulcers (DFUs), says Dr Rastogi, are common but not discussed as much as a severe complication of diabetes. The prevalence of DFUs is between 1.3 and 12 per cent in various countries. Indians rarely take up issues pertaining to DFU seriously because of socio-cultural beliefs, delayed health-seeking behaviour and out-of-pocket expenditure. “Around one in five patients with diabetes is likely to develop foot ulcers. Foot complications, including DFUs, substantially contribute to diabetes-related mortality because of infections and stubborn wounds that require amputation. In a large study, spanning several centres across the country, we found that a person with DFU is three times more likely to die earlier (mainly because of cardiac issues) than a routine diabetic person without ulcer. Also, mortality due to foot ulcers is higher than certain cancers,” explains Dr Rastogi.
Even with the best of therapy, only 30 per cent of wounds associated with diabetes heal despite several advanced treatment modalities. These include advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation and negative pressure wound therapy. Also, the ulcer recurrence rate is 40 to 70 per cent. “The main goal is prevention of foot ulcers through routine screening and regular checkups. However, after formation of ulcer, complete wound healing is the primary outcome measure. Additionally, many of these patients harbour other complications, including cardiac, renal and infectious issues which need comprehensive management,” he says.
As for challenges faced in routine management of diabetic foot disease and associated ulcers, Dr Rastogi explains that in India, around 70 per cent of the population lives in rural areas but 80 per cent of the doctors provide services in urban areas. Thus, the rural population has minimal access to good healthcare services. Foot self-care education and examination are essential for preventing foot complications, but a lack of trained podiatrist staff in India causes an increased prevalence of diabetes foot-related complications.
Elaborating on the premise of the study, Dr Rastogi says that even with the best standards of care, only one in three diabetic foot wounds heals and hence there is a need for research and innovation to develop newer treatment options. “We conducted the first proof of concept study in-vitro in rats and guinea pigs to demonstrate how esmolol can improve migration of certain cells and growth factors around the wound to hasten ulcer healing. We also demonstrated that esmolol improves collagen content that is required for wound closure and showed its safety. Subsequently, we performed the first human study in the world with topical esmolol and demonstrated its safety at 14 per cent gel concentration.”
Heart-valve technology
Transcatheter Aortic Valve Replacement
January 7, 2020: The Times of India
Made in India heart valve technology can keep your heart beating strong!
Sarah*, a French woman was 83, when she had been advised that she had only a few hours to live. However, she decided to opt for a unique, cutting-edge procedure that could grant her a fresh lease of life. Within a year of the procedure, she traveled to America to achieve her dream of addressing a large gathering at a prestigious conference. Now, at age of 90, she continues to live an active and normal life.
What threatened her life, and which procedure saved her?
Sarah had a medical condition called Aortic Valve Stenosis (AS). The valve in her heart, which ensures normal blood-flow from the heart to the body, had narrowed with advancing age. This made it extremely difficult for her to carry out simple daily activities. She often felt out of breath. There was a tightness in her chest, frequently accompanied by pain. She felt dizzy even with brief movements. In short, living had become a struggle. Medication did not provide any significant relief. The other available treatment was an open heart surgery for valve replacement. But that was an extremely risky option due to her age, the complex nature of the surgery, the long hospital stay, and high chance of complications post-surgery, especially during the recovery period of 10-12 months. Given these risks, surgical teams in Paris had refused to perform a surgical valve replacement. At this point, Sarah consulted Dr. Alain Cribier, Professor emeritus at the Department of Cardiology, University Hospital Charles Nicolle in Rouen (France). Dr. Cribier suggested that she consider the option of an innovative non-surgical procedure called Transcatheter Aortic Valve Replacement (TAVR).
The procedure entailed a small cut in the groin or chest area to pass a catheter tube to replace the diseased valve with a bioprosthetic valve. There would be no need to cut open the chest cavity nor any need for general anesthesia as is usually required for surgery. The TAVR procedure would be performed over 30-40 minutes, and her recovery would be swift, requiring hospital stay of only 3 to 5 days.
Dr. Alain Cribier, who has established TAVR therapy worldwide shares, “In the 17 years since I performed the first TAVR procedure in 2002, more than 350,000 patients in 65 countries have undergone this revolutionary procedure. In the beginning, when the technology was new, TAVR was recommended only to high-risk patients who couldn’t undergo open heart surgery. However, advances in technology and availability of long-term safety data have resulted in TAVR becoming a routine and preferred procedure today. It can now be applied in a very high number of patients. In the year 2018, 68,000 TAVR procedures were performed in USA and 18,000 in France. In the hospital where I practice, we are conducting 5-10 procedures a week.”
However, the Indian scenario is quite different, as the procedure has not reached many patients who can benefit from it. Approximately 300,000 severe AS patients cannot undergo open-heart surgery because of old age, frailty, or other medical reasons. [1] TAVR therapy can be life saving for these patients. Yet, less than 1,000 TAVR procedures are being performed on an annual basis in India. Many lives can be saved if awareness of this promising procedure is raised.
Meril Life Sciences, a Gujarat-based medical device manufacturer with a global footprint spread over 150 countries, is committed to bring about this change. Known for indigenous research and development, and a broad portfolio of stents, surgical and orthopedic devices, this company has recently launched an advanced bioprosthetic valve. The new valve has a unique ‘hybrid honeycomb’ design that allows doctors to precisely place the device at its natural position. This reduces the need for a new pacemaker, otherwise required in 10 to 30 percent of patients receiving other available valve technologies.
Results of the MyVal-1 scientific study , published in the Euro Intervention Journal, prove the benefits of this valve technology. There was high device success, low incidence of stroke, and low requirement of a permanent pacemaker implant post-procedure. Significant improvement in patient heart function and quality of life was also observed. After CE approval of this valve technology, hospitals in Europe have commenced using the valve due to these benefits.
“Companies like Meril Life Sciences are helping bring the benefits of non-surgical TAVR therapy to more patients by training Interventional Cardiologists in India. They have also made this cutting-edge treatment more accessible and more affordable to Indian patients,” sums up Dr. Alain Cribier.
As these efforts come to fruition, many more Indian patients will have the chance to live life to their heart’s content, just as Sarah did.
- Patient name edited to ensure privacy.
[1] Based on incidence data applied to the Indian population, J Am Coll Cardiol. 2013;62(11):1002-1012. doi:10.1016/j.jacc.2013.05.015
Note: This article is intended for educational purposes only, and does not seek to serve as a substitute for medical advice, nor to advocate a treatment or device in use for patients. Only qualified medical experts can provide advice regarding your individual treatment. Please consult your doctor for more details.
Insulin
That can be kept without refrigeration/ 2021
Jhimli MukherjeePandey, Sep 24, 2021: The Times of India
From: Jhimli MukherjeePandey, Sep 24, 2021: The Times of India
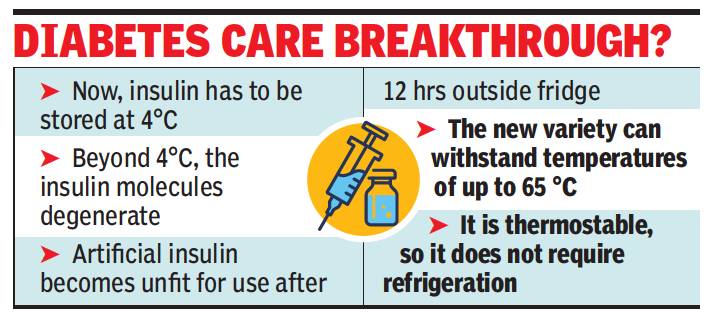
A team of scientists, including two from Kolkata, have developed a “thermostable” variety of insulin, which eliminates the need to keep it refrigerated. The development is being seen as a breakthrough in scientific circles, with portability being the biggest hurdle for insulin-dependent diabetics.
The research has been led by two scientists of the Bose Institute and the Indian Institute of Chemical Biology (IICB) here and two others from the Indian Institute of Chemical Technology (IICT), Hyderabad.
“You will be able to keep it outside the refrigerator for as long as you want, something that will help diabetes patients across the world because carrying insulin along with them was considered impossible all this while,” said Subhrangsu Chatterjee, a faculty member at Bose Institute.
“Though for the moment we are calling it ‘insulock’, we are in the process of appealing to the department of science and technology (DST) to name it after Acharya Jagadish Chandra Bose,” he added.
The research has been lauded by iScience, a science journal of international repute. Chatterjee and Partha Chakrabarti, a faculty member at IICB, along with IICT’s B Jagadeesh and J Reddy, were able to introduce a matrix of four amino acid peptide molecules inside insulin molecules, which prevented solidification of the insulin molecules even when not refrigerated. While insulin needs to be kept at an ideal temperature of 4 °C now, this new variety would be able to withstand a temperature of up to 65 °C, the researchers claim. The four-year-long research into the structural design of insu-lock was funded jointly by DST and CSIR.
Chatterjee said, after 12 hours of staying at normal room temperature, it reaches a stage where it becomes unfit for use. “That is why it is so expensive. We are hopeful that DST and CSIR will now help us go for corporate tie-ups for mass production,” he told TOI.
Migraine
2023: wearable device for drug-free relief
Nov 17, 2023: The Times of India
At ₹14k, new wearable device offers drug-free migraine relief
Swati.Bharadwaj@timesgroup.com
Hyderabad : Treatment of acute migraine need no longer be a headache for doctors and patients alike. Hyderabad-based Dr Reddy’s Laboratories is rolling out a US FDA approved wearable therapy device called Nerivio that the firm calls a pathbreaking drug-free cure for migraine.
The prescription-based non-invasive device meant to be worn on upper arm has been approved for adults & adolescents aged 12-plus diagnosed with acute migraine, with or without aura. The company says wearing the device helps prevent migraine.
Nerivio, priced at Rs 14,000-16,000, uses the remote electrical neuromodulation mechanism to activate conditioned pain modulation by stimulating nerve endings.
“This initiates a natural pain-relieving process in the brainstem, causing a global effect of pain inhibition that affects the original source of migraine pain in the head,” states the company.
The device comes with inbuilt 18 sessions of 45 minutes each, and has to be used within 60 minutes of onset of headache for treatment of acute migraine or every alternate day for migraine prevention.
After 18 sessions, the device has to be discarded for a fresh one. A mobile app controls device’s intensity levels.
The app is equipped with an interactive migraine diary that can be used to log symptoms, track responses and share insightful analytics. It also has an interactive GIER (guided, imagery, education and relaxation) protocol that can significantly increase response rates if used with the device, the company says.
MV Ramana, CEO, branded markets (India & emerging markets) at Dr Reddy’s, said the device marks the firm’s foray into digital therapeutics.
The launch comes after Dr Reddy’s entered into an exclusive agreement with Israeli digital therapeutics company Theranica Bio-electronics, which is developing advanced neuromodulation devices for migraine and management of other pain conditions.
Patents filed
2020-2022
Rupali Mukherjee, April 25, 2023: The Times of India

From: Rupali Mukherjee, April 25, 2023: The Times of India
Mumbai: Highlighting the innovation theme during the coronavirus, India has emerged as one of the top 10 patent filers globally in three different categories — therapeutics, vaccines as well as traditional medicines related to Covid.
India ranked fourth among therapeutic patent filers, with 195 applications, according to data culled from the Covid ‘patent landscape’ report by the World Intellectual Property Organization (WIPO).
Under traditional medicines — those made from herbs, animals or natural sources — India stood second with 59 filers, following China’s 341 applications. These also seem to have had a role to play in the fight against Covid, the report says, adding 10% of filings disclosed the use of traditional medicine in treating Covid.
Further, in terms of vaccines, the Indian Patent Office received 65 vaccine patent applications, the seventh highest recipient globally.
The findings were recently made available, based on a comprehensive review by WIPO of patenting activity from January 2020 through September 2022. They showed innovators filed thousands of patents for new technologies to battle the pandemic, with the majority related to therapies.
The report found 7,758 patent filings on technologies related to Covid in general, including 1,298 related to vaccine development and 4,787 to therapeutics. Patenting activity related to Covid outpaced that of other recent viru- ses and illnesses, such as influenza and SARS, both in volume and speed of filing, the analysis added.
China is the leading origin of patent filings related to vaccines as well as therapeutics, at 573 and 1,850 applications respectively. Other top applicant locations are the US, Germany, Korea and Russia. In therapeutics, the US, Korea, India and Germany as the other top applicant locations.
Just 5% of vaccine patents in the dataset emphasised booster use. This is in sharp contrast to the extensive media discussion, government recommendations and policy debates around the availability and use of boosters.
Rare diseases
2023: four medicines approved
Nov 25, 2023: The Times of India
Govt approves 4 home-grown, cheaper drugs for rare diseases
TIMES NEWS NETWORK
New Delhi : In a major relief for rare disease patients, the government said on Friday it has approved the marketing of four drugs manufactured by Indian pharmaceutical firms that are drastically cheaper than their imported versions.
For example, while the annual cost of the imported Nitisinone capsule that is used in the treatment of Tyrosinemia Type 1 comes to Rs 2.2 crore, the domestically manufactured capsules will now be available for just Rs 2.5 lakh.Tyrosinemia Type 1 is a rare disease that affects one in a lakh population.
Similarly, while the cost of the imported Eliglustat capsules that is used in the treatment of Gaucher’s disease comes at Rs 1.8-3.6 crore per annum, the domestically manufactured capsules will now be available for just Rs 3-6 lakh per annum. The cost of the imported Trientine capsules used in treatment of Wilson’s disease comes to Rs 2.2 crore per annum but with it being manufactured indigenously it will be available Rs 2.2 lakh.
The cost of the imported Cannabidiol (oral solution) used in treatment of Dravet-Lennox Gastaut Syndrome comes to Rs 7-34 lakh per annum but with it being manufactured indigenously it will be available for Rs 1-5 lakh per annum, offi cials said.The commercial supply of Hydroxyurea Syrup used in the treatment of sickle cell anemia is likely to begin by March 2024 and the tentative price would be Rs 405 per bottle. The cost of this oral suspension is $840 (Rs 70,000) per 100 ml. All these drugs were not manufactured in the country till now.
“The exercise to encourage Indian companies to manufacture generic versions of drugs used in treatment of the rare diseases, also referred to as the orphan drugs, started in July 2022 and discussions were held with academia, pharma industries, organisations, CDSCO, Department of Pharmaceuticals after which 13 rare diseases were prioritised along with sickle cell anaemia. After which interactions were held with drug manufacturers and the Drugs Controller General of India after which these drugs were approved and prices were slashed,”said an official.
Trientine/ D-Pencillamine
MSN Laboratories/ 2019
Over two years after patients of a rare genetic condition called Wilson’s Disease suffered due to acute shortage of a life-saving drug called D-Pencillamine across the country, there is finally a reason to cheer. An Indian company has begun manufacturing a drug called Trientine, which is considered superior to D-Pencillamine due to fewer side-effects, at a fraction of its international price.
What makes this new drug launch an emotional event is the behind-the-scenes lobbying by a group of parents, an NGO and a doctor from Mumbai who worked to convince Hyderabad-based MSN Laboratories to take up the manufacture of Trientine.
At a time when stories of the greed of pharmaceutical companies abound, this is a positive instance of pharmaceutical support. Dr Aabha Nagral, a hepatologist who set up NGO Children’s Liver Foundation, said a couple of children had to stop treatment when the drug shortage occurred in 2016.
‘Firm involved in social work; not driven by profits’
We organised a picnic for our patient group where two youngsters shared that they had no drugs at that time and were worried about the future,” said Dr Nagral, who has treated approximately 100 Wilson’s Disease patients in her care at any given time.
The Ranes from Vile Parle, too, recall how their daughter Gauri was hospitalised a few times during the shortage. “Fluid would get accumulated in her abdomen, requiring hospitalisation. It was a traumatising time as her liver was failing quickly,” said Satish Rane. Luckily, she got a cadaveric liver donation and is doing well since the transplant a year ago.
Rajesh S (name changed), a 45-year-old research scientist, was diagnosed with Wilson’s Disease a year back. “We had heard of shortages and expensive medicines costing almost Rs 1 lakh for 100 tablets but we now pay Rs 16,000 for 100 tablets,” said his wife.
The turnaround took months of gathering information and planning. After the shortage was fixed by the Union government’s directive to pharmaceutical companies in August 2016, Dr Nagral and the patient group actively started to seek information about Trientine. They heard from pharmaceutical agents that the active ingredient for Trientine was manufactured in an Indian company and exported to multinational companies located overseas.
“We applied through RTI to the Union ministry of information on the manufacture of Trientine and D-Pencillamine, but got little,” said Dr Nagral. That is when they began to directly appeal to pharmaceutical companies for help. MSN Laboratories, which has been manufacturing active pharmaceutical ingredients for export purposes for years, decided to help out. Ajit Bhadauria of MSN Laboratories said the company didn’t take up the manufacture of Trientine for profits. “It is purely a part of our social work as a pharmaceutical company,” he said.
Vaccines
Pneumonia vaccine/ 2020
Sushmi Dey, India gets its first desi pneumonia vaccine, July 16, 2020: The Times of India
NEW DELHI: India has got its first locally developed pneumonia vaccine, reducing its dependence on imports for immunisation against a disease that contributes to most deaths in children under five years of age. The Drugs Controller General of India (DCGI) on Tuesday approved Pneumococcal Polysaccharide Conjugate Vaccine developed by Serum Institute of India. The Pune-based company can now manufacture the vaccine in India for use.
This vaccine is used for active immunisation against invasive disease and pneumonia caused by “streptococcus pneumonia” in infants. “Having an indigenous pneumococcal vaccine will be a game-changer in our endeavour to reduce child mortality. Pneumonia is the most important cause of child deaths, and pneomococci are responsible for half of serious pneumonias. India’s vaccine is a boon for our country and the world,” says Dr V K Paul, member -health at NITI Aayog.
India accounted for the second highest number of deaths in under-five children in 2018 because of pneumonia. According to Unicef, India reported 1,27,000 under five deaths due to pneumonia in 2018. Earlier, the demand of this vaccine was met substantially by licensed importers in the country since the manufacturers were all vaccine companies based outside. The regulatory approval is based on clinical evidence secured by the company after conducting Phase I, Phase II and Phase III clinical trials of the vaccine. Serum Institute has conducted these trials in India as well as in Gambia.
While immunisation against the disease has improved in India led by government initiatives and awareness programmes, many children — mainly female children — are still left out of the coverage. Experts say a locally made vaccine is likely to make the vaccine more accessible and affordable.
India accounted for the second highest number of deaths in under-five children in 2018 because of pneumonia. According to Unicef, India reported 1,27,000 under five deaths due to pneumonia in 2018.
Covaxin/ 2021
Swati Bharadwaj, January 23, 2021: The Times of India
Amid efficacy row, Covaxin gets thumbs-up from Lancet
HYDERABAD: Amid a raging controversy over the efficacy of Covaxin, India’s first indigenous Covid-19 vaccine, reputed medical journal the Lancet Infectious Diseases said the vaccine produced tolerable safety outcomes and enhanced immune responses in its Phase 1 trials.
With this, Covaxin has become the first Covid-19 vaccine from India to have its data published in Lancet, said Suchitra Ella, joint managing director of Bharat Biotech, which is developing the vaccine in association with the Indian Council for Medical Research and the National Institute of Virology, Pune.
"BBV152 (codename for Covaxin) induced binding and neutralising antibody responses and with the inclusion of the Algel-IMDG adjuvant, this is the first inactivated SARS-CoV-2 vaccine that has been reported to induce a Th1-biased response," said the journal.
Covaxin was well tolerated in all dose groups, with no vaccine-related serious adverse events and the sole serious adverse event was not causally associated with the vaccine, the journal said.
According to biotech industry experts, any vaccine causes some discomfort like pain or fever but it is said to be tolerable when it is resolved without any treatment and is considered safe. A serious adverse side-effect is one which takes a lot of time and effort to treat and causes partial damage to the subject.
The biggest criticism against Covaxin was that none of its data was in the public domain when it was given emergency-use authorisation. Sources said Bharat Biotech is hoping to counter the critics with the Lancet piece.
Terming it as "international validation of Indian innovation", Ella tweeted: "Extremely proud to announce Phase 1 clinical trial studies of Covaxin published in the most prestigious medical journal of The Lancet Infectious Diseases."
Ella also said that 13,000 volunteers (out of 25,800) have been successfully administered the second dose of Covaxin in Phase 3 trials.
Lancet said all adverse events observed in the Phase 1 trials were mild or moderate and were more frequent after the first dose than the second. No significant differences were observed between the vaccinated and control groups, it added.
The most common adverse event was pain at the injection site, followed by headache, fatigue and fever. "The overall incidence of solicited local and systemic adverse events in this study was 14-21% in all vaccine-treated groups, which is noticeably lower than the rates for other SARS-CoV-2 vaccine platform candidates," it said.
As many as 44 unsolicited adverse events were reported by 24 (6%) of the 375 participants. The healthy volunteers, in the 18-55 years age group that were enrolled in the trials conducted in July-August 2020, were administered the two doses 14 days apart.
Of those enrolled, 100 each were randomly assigned to the three vaccine groups, and 75 were randomly assigned to the control group (Algel only).
Apart from carrying Covaxin Phase 1 trial data, the Lancet carried another article saying that the indigenous Indian vaccine was a "welcome addition to the Covid-19 vaccine landscape". But it also said that it would wait for Phase 3 trial results.
Pointing out that mathematical models indicate there will not be an adequate supply of vaccines available to cover the global population until 2023, the article said: "Bharat Biotech, experienced with developing and distributing vaccines to LMICs (low & middle-income countries) is poised to bridge the vaccine disparity gap using the well-established inactivated whole-virus vaccine platform."
However, it said that until questions about BBV152’s efficacy and ability to subvert a Th2 response are answered by a more diverse multinational Phase 3 trial to comprehensively assess efficacy and long-term safety, "we will wait with cautious optimism on this vaccine candidate poised to bolster worldwide equitable access to Covid-19 prevention".
See also
Drugs and Pharmaceuticals: India
Drugs, pharmaceuticals, medical devices made by Indians